Clinical Significance and Prognostic Relevance of Microsatellite Instability in Sporadic Colorectal Cancer Patients
Abstract
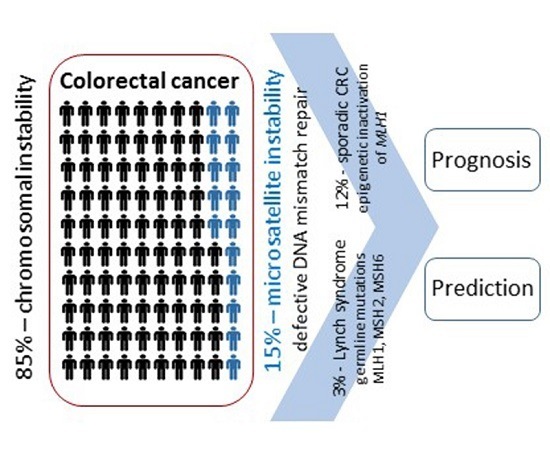
:1. Introduction
2. Microsatellite Instability (MSI) Status Assessment
3. MSI Status and Colorectal Cancer (CRC) Prognosis
4. MSI Status and 5-Fluorouracil (FU) Chemotherapy Response
5. Predictive Value of MSI Status in II and III CRC
6. Predictive Value of MSI Status in Metastatic CRC
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Thomas, M.L.; Hewett, P.J.; Ruszkiewicz, A.R.; Moore, J.W. Clinicopathological predictors of benefit from adjuvant chemotherapy for stage C colorectal cancer: Microsatellite unstable cases benefit. Asia Pac. J. Clin. Oncol. 2015, 11, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Adlard, J.W.; Richman, S.D.; Seymour, M.T.; Quirke, P. Prediction of the response of colorectal cancer to systemic therapy. Lancet Oncol. 2002, 3, 75–82. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 1, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.R.; Bosman, F.T.; Boffetta, P. Carcinoma of the colon and rectum. In WHO Classification of Tumor of the Digestive System, 4th ed.; Bosman, F.T., Carneiro, F., Hruban, R.H., Eds.; IARC Press: Lyon, France, 2010; pp. 133–138. [Google Scholar]
- Saridaki, Z.; Souglakos, J.; Georgoulias, V. Prognostic and predictive significance of MSI in stages II/III colon cancer. World Gastroeterol. 2014, 20, 6809–6814. [Google Scholar] [CrossRef] [PubMed]
- Iachetta, F.; Domati, F.; Reggiani-Bonetti, L.; Barresi, V.; Magnani, G.; Marcheselli, L.; Cirilli, C.; Pedroni, M. Prognostic relevance of microsatellite instability in pT3N0M0 colon cancer: A population-based study. Intern. Emerg. Med. 2016, 11, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Sargent, D.J.; Marsoni, S.; Monges, G.; Thibodeau, S.N.; Labianca, R.; Hamilton, S.R.; French, A.J.; Kabat, B.; Foster, N.R.; Torri, V.; et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluoruracil-based adjuvant therapy in colon cancer. J. Clin. Oncol. 2010, 28, 3219–3226. [Google Scholar] [CrossRef] [PubMed]
- Quasar Collaborative Group; Gray, R.; Barnwell, J.; McConkey, C.; Hills, R.K.; Williams, N.S.; Kerr, D.J. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007, 370, 2020–2029. [Google Scholar] [PubMed]
- Turyn, J. Mikrosatelitarny DNA. Postepy Biochem. 2004, 50, 198–208. [Google Scholar] [PubMed]
- Wierzbicki, P.M.; Adrych, K.; Kartanowicz, D.; Wypych, J.; Stanisławowski, M.; Dobrowolski, S.; Chybicki, J.; Zwolińska-Wcisło, M.; Celiński, K.; Korybalski, B.; et al. Microsatellite instability status in inflammatory bowel disease and colorectal cancer. Ann. Acad. Med. Gedan. 2009, 39, 163–171. [Google Scholar]
- Gatalica, Z.; Vranic, S.; Xiu, J.; Swensen, J.; Reddy, S. High microsatellite instability (MSI-H) colorectal carcinoma: A brief review of predictive biomarkers in the era of personalized medicine. Fam. Cancer 2016, 15, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Jass, J.R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007, 50, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.S.; Chung, D.C. The chromosomal instability pathway in colon cancer. Gastroenterology 2010, 138, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Umar, A.; Polyak, K.; Graff, J.R.; Ahuja, N.; Issa, J.P.; Markowitz, S.; Willson, J.K.; Hamilton, S.R.; Kinzler, K.W.; et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc. Natl. Acad. Sci. USA 1998, 95, 6870–6875. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Chi, Y.; Chen, W.; Chen, X.; Wei, P.; Sheng, W.; Zhou, X.; Shi, D. Immunohistochemistry and microsatellite instability analysis in molecular subtyping of colorectal carcinoma based on mismatch repair competency. Int. J. Clin. Exp. Med. 2015, 8, 20988–21000. [Google Scholar] [PubMed]
- Cunningham, J.M.; Kim, C.Y.; Christensen, E.R.; Tester, D.J.; Parc, Y.; Burgart, L.J.; Halling, K.C.; McDonnell, S.K.; Schaid, D.J.; Walsh Vockley, C.; et al. The frequency of hereditary defective mismatch repair in a prospective series of unselected colorectal carcinomas. Am. J. Hum. Genet. 2001, 69, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.M.; Christensen, E.R.; Tester, D.J.; Kim, C.Y.; Roche, P.C.; Burgart, L.J.; Thibodeau, S.N. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res. 1998, 58, 3455–3460. [Google Scholar] [PubMed]
- Kane, M.F.; Loda, M.; Gaida, G.M.; Lipman, J.; Mishra, R.; Goldman, H.; Jessup, J.M.; Kolodner, R. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997, 57, 808–811. [Google Scholar] [PubMed]
- Colussi, D.; Brandi, G.; Bazzoli, F.; Ricciardiello, L. Molecular pathways involved in colorectal cancer: Implications for disease behavior and prevention. Int. J. Mol. Sci. 2013, 14, 16365–16385. [Google Scholar] [CrossRef]
- Mao, L.; Lee, D.J.; Tockman, M.S.; Erozan, Y.S.; Askin, F.; Sidransky, D. Microsatellite alterations as clonal markers for the detection of human cancer. Proc. Natl. Acad. Sci. USA 1994, 91, 9871–9875. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, X.; Tian, L.; Che, G. Microsatellite alteration in multiple primary lung cancer. J. Thorac. Dis. 2014, 6, 1499–1505. [Google Scholar] [PubMed]
- Kubecek, O.; Trojanova, P.; Molnarova, V.; Kopecky, J. Microsatellite instability as a predictive factor for immunotherapy in malignant melanoma. Med. Hypotheses 2016, 93, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Karpińska-Kaczmarczyk, K.; Lewandowska, M.; Ławniczak, M.; Białek, A.; Urasińska, E. Expression of mismatch repair proteins in early and advanced gastric cancer in Poland. Med. Sci. Monit. 2016, 22, 2886–2892. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, N.; Mathew, B.B.; Jatawa, S.K.; Tiwari, A. Genetic instability in urinary bladder cancer: An evolving hallmark. J. Postgrad. Med. 2013, 59, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Lemon, S.J.; Wang, S.; Franklin, B.; Watson, P.; Knezetic, J.A.; Bewtra, C.; Lynch, H.T. Microsatellite instability and expression of MLH1 and MSH2 in normal and malignant endometrial and ovarian epithelium in hereditary nonpolyposis colorectal cancer family members. Cancer Genet. Cytogenet. 1999, 112, 2–8. [Google Scholar] [CrossRef]
- McMeekin, D.S.; Tritchler, D.L.; Cohn, D.E.; Mutch, D.G.; Lankes, H.A.; Geller, M.A.; Powell, M.A.; Backes, F.J.; Landrum, L.M.; Zaino, R.; et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: An NRG oncology/gynecologic oncology group study. J. Clin. Oncol. 2016, 34, 3062–3068. [Google Scholar] [CrossRef] [PubMed]
- Segev, Y.; Pal, T.; Rosen, B.; McLaughlin, J.R.; Sellers, T.A.; Risch, H.A.; Zhang, S.; Sun, P.; Narod, S.A.; Schildkraut, J. Risk factors for ovarian cancers with and without microsatellite instability. Int. J. Gynecol. Cancer 2014, 24, 664–669. [Google Scholar] [CrossRef]
- Loughrey, M.B.; Waring, P.M.; Tan, A.; Trivett, M.; Kovalenko, S.; Beshay, V.; Young, M.A.; McArthur, G.; Boussioutas, A.; Dobrovic, A. Incorporation of somatic BRAF mutation testing into an algorithm for the investigation of hereditary non-polyposis colorectal cancer. Fam. Cancer 2007, 6, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, G.; Southward, K.; Handley, K.; Magill, L.; Beaumont, C.; Stahlschmidt, J.; Richman, S.; Chambers, P.; Seymour, M.; Kerr, D.; et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J. Clin. Oncol. 2011, 29, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, P.; Kuchiba, A.; Imamura, Y.; Liao, X.; Yamauchi, M.; Nishihara, R.; Qian, Z.R.; Morikawa, T.; Shen, J.; Meyerhardt, J.A.; et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J. Natl. Cancer Inst. 2013, 105, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Venderbosch, S.; Nagtegaal, I.D.; Maughan, T.S.; Smith, C.G.; Cheadle, J.P.; Fisher, D.; Kaplan, R.; Quirke, P.; Seymour, M.T.; Richman, S.D.; et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 2014, 20, 5322–5330. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Svrcek, M.; Dreyer, C.; Cervera, P.; Duval, A.; Pocard, M.; Fléjou, J.F.; de Gramont, A.; André, T. New therapeutic opportunities based on DNA mismatch repair and BRAF status in metastatic colorectal cancer. Curr. Oncol. Rep. 2016, 18, 18. [Google Scholar] [CrossRef]
- Roth, A.D.; Tejpar, S.; Delorenzi, M.; Yan, P.; Fiocca, R.; Klingbiel, D.; Dietrich, D.; Biesmans, B.; Bodoky, G.; Barone, C.; et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: Results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J. Clin. Oncol. 2010, 28, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Umeda, Y.; Nagasaka, T.; Mori, Y.; Sadamori, H.; Sun, D.S.; Shinoura, S.; Yoshida, R.; Satoh, D.; Nobuoka, D.; Utsumi, M.; et al. Poor prognosis of KRAS or BRAF mutant colorectal liver metastasis without microsatellite instability. J. Hepatobiliary Pancreat. Sci. 2013, 20, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Koopman, M.; Kortman, G.A.; Mekenkamp, L.; Ligtenberg, M.J.; Hoogerbrugge, N.; Antonini, N.F.; Punt, C.J.; van Krieken, J.H. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br. J. Cancer 2009, 100, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Morris, M.; Idrees, K.; Gimbel, M.I.; Rosenberg, S.; Zeng, Z.; Li, F.; Gan, G.; Shia, J.; LaQuaglia, M.P.; et al. Colorectal cancer in the very young: A comparative study of tumor markers, pathology and survival in early onset and adult onset patients. J. Pediatr. Surg. 2016, 51, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Llosa, N.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Freeman, G.J. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov. 2015, 5, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, D.; Lyerly, H.K.; Morse, M.A. Deficient Mismatch Repair and the role of immunotherapy in metastatic colorectal cancer. Curr. Treat. Opt. Oncol. 2016, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Boissière-Michot, F.; Lazennec, G.; Frugier, H.; Jarlier, M.; Roca, L.; Duffour, J.; Du Paty, E.; Laune, D.; Blanchard, F.; Le Pessot, F.; et al. Characterization of an adaptive immune response in microsatellite-instable colorectal cancer. Oncoimmunology 2014, 3, e29256. [Google Scholar] [CrossRef] [PubMed]
- Belov, L.; Zhou, J.; Christopherson, R.I. Cell surface markers in colorectal cancer prognosis. Int. J. Mol. Sci. 2010, 12, 78–113. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colon Cancer (Version 2.2016). Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed on 5 January 2017).
- Association for Molecular Pathology. Available online: Https://www.amp.org/committees/clinical_practice/AMPclinicalpracticeguidelines.cfm (accessed on 5 January 2017).
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; Rodriguez-Bigas, M.A.; Fodde, R.; Ranzani, G.N.; et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar] [PubMed]
- Lindor, N.M.; Burgart, L.J.; Leontovich, O.; Goldberg, R.M.; Cunningham, J.M.; Sargent, D.J.; Walsh-Vockley, C.; Petersen, G.M.; Walsh, M.D.; Leggett, B.A.; et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J. Clin. Oncol. 2002, 20, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Gafà, R.; Maestri, I.; Santini, A.; Matteuzzi, M.; Cavazzini, L. Immunohistochemical pattern of MLH1/MSH2 expression is related to clinical and pathological features in colorectal adenocarcinomas with microsatellite instability. Mod. Pathol. 2002, 5, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.G.; Robertson, D.; Houlston, R.S. Immunohistochemistry for MSH2 and MLH1: A method for identifying mismatch repair defi cient colorectal cancer. J. Clin. Pathol. 2001, 54, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Ohrling, K.; Edler, D.; Hallström, M.; Ragnhammar, P. Mismatch repair protein expression is an independent prognostic factor in sporadic colorectal cancer. Acta Oncol. 2010, 49, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, S.N.; Bren, G.; Schaid, D. Microsatellite instability in cancer of the proximal colon. Science 1993, 260, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Halling, K.C.; French, A.J.; McDonnell, S.K.; Burgart, L.J.; Schaid, D.J.; Peterson, B.J.; Moon-Tasson, L.; Mahoney, M.R.; Sargent, D.J.; O’Connell, M.J.; et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J. Natl. Cancer Inst. 1999, 91, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Hubner, R.; Houlston, R.S. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 2005, 23, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Søreide, K.; Slewa, A.; Stokkeland, P.J.; van Diermen, B.; Janssen, E.A.; Søreide, J.A.; Baak, J.P.; Kørner, H. Microsatellite instability and DNA ploidy in colorectal cancer: Potential implications for patients undergoing systematic surveillance after resection. Cancer 2009, 115, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Søreide, K.; Nedrebø, B.S.; Søreide, J.A.; Slewa, A.; Kørner, H. Lymph node harvest in colon cancer: Influence of microsatellite instability and proximal tumor location. World J. Surg. 2009, 33, 2695–2703. [Google Scholar] [CrossRef] [PubMed]
- Belt, E.J.; te Velde, E.A.; Krijgsman, O.; Brosens, R.P.; Tijssen, M.; van Essen, H.F.; Stockmann, H.B.; Bril, H.; Carvalho, B.; Ylstra, B.; et al. High lymph node yield is related to microsatellite instability in colon cancer. Ann. Surg. Oncol. 2012, 19, 1222–1230. [Google Scholar] [CrossRef]
- Mohan, H.M.; Ryan, E.; Balasubramanian, I.; Kennelly, R.; Geraghty, R.; Sclafani, F.; Fennelly, D.; McDermott, R.; Ryan, E.J.; O’Donoghue, D.; et al. Microsatellite instability is associated with reduced disease specific survival in stage III colon cancer. Eur. J. Surg. Oncol. 2016, 42, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Buckowitz, A.; Knaebel, H.P.; Benner, A.; Bläker, H.; Gebert, J.; Kienle, P.; von Knebel Doeberitz, M.; Kloor, M. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br. J. Cancer 2005, 92, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Malesci, A.; Laghi, L.; Bianchi, P.; Delconte, G.; Randolph, A.; Torri, V.; Carnaghi, C.; Doci, R.; Rosati, R.; Montorsi, M.; et al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin. Cancer Res. 2007, 13, 3831–3939. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Ahn, J.B.; Jung, M.; Beom, S.H.; Kim, C.; Kim, J.H.; Heo, S.J.; Park, H.S.; Kim, J.H.; Kim, N.K.; et al. Effects of microsatellite instability on recurrence patterns and outcomes in colorectal cancers. Br. J. Cancer 2016, 115, 25–33. [Google Scholar] [CrossRef] [PubMed]
- MacQuarrie, E.; Arnason, T.; Gruchy, J.; Yan, S.; Drucker, A.; Huang, W.Y. Microsatellite instability status does not predict total lymph node or negative lymph node retrieval in stage III colon cancer. Hum. Pathol. 2012, 43, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Focke, F.; Saito, Y.; Schuermann, D.; Lettieri, T.; Selfridge, J.; Schär, P. Base excision by thymine DNA glycosylase mediates DNA-directed cytotoxicity of 5-fluorouracil. PLoS Biol. 2009, 7, e91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guastadisegni, C.; Colafranceschi, M.; Ottini, L.; Dogliotti, E. Microsatellite instability as a marker of prognosis and response to therapy: A meta-analysis of colorectal cancer survival data. Eur. J. Cancer 2010, 46, 2788–2798. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Baerenfaller, K.; Jiricny, J. 5-Fluorouracil is efficiently removed from DNA by the base excision and mismatch repair systems. Gastroenterology 2007, 133, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Carethers, J.M.; Chauhan, D.P.; Fink, D.; Nebel, S.; Bresalier, R.S.; Howell, S.B.; Boland, C.R. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology 1999, 117, 123–131. [Google Scholar] [CrossRef]
- Arnold, C.N.; Goel, A.; Boland, C.R. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int. J. Cancer 2003, 106, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.; Wagner, M.W.; Hwang, H.S.; Kinsella, T.J.; Boothman, D.A. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res. 2001, 61, 5193–5201. [Google Scholar] [PubMed]
- Elsaleh, H.; Joseph, D.; Grieu, F.; Zeps, N.; Spry, N.; Iacopetta, B. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet 2000, 355, 1745–1750. [Google Scholar] [CrossRef]
- Lukish, J.R.; Muro, K.; DeNobile, J.; Katz, R.; Williams, J.; Cruess, D.F.; Drucker, W.; Kirsch, I.; Hamilton, S.R. Prognostic significance of DNA replication errors in young patients with colorectal cancer. Ann. Surg. 1998, 227, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, A.; Mecklin, J.P.; Jarvinen, H.; Aaltonen, L.A.; Joensuu, H. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology 2000, 119, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.P.; Colangelo, L.H.; Wieand, H.S.; Paik, S.; Kirsch, I.R.; Wolmark, N.; Allegra, C.J. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: A National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J. Clin. Oncol. 2007, 25, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Wu, T.T.; Catalano, P.J.; Ueki, T.; Satriano, R.; Haller, D.G.; Benson, A.B., 3rd; Hamilton, S.R. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2001, 344, 1196–1206. [Google Scholar] [CrossRef]
- Barratt, P.L.; Seymour, M.T.; Stenning, S.P.; Georgiades, I.; Walker, C.; Birbeck, K.; Quirke, P. DNA markers predicting benefit from adjuvant fluorouracil in patients with colon cancer: A molecular study. Mech. Dis. 2002, 360, 1381–1387. [Google Scholar] [CrossRef]
- Ribic, C.M.; Sargent, D.J.; Moore, M.J.; Thibodeau, S.N.; French, A.J.; Goldberg, R.M.; Hamilton, S.R.; Laurent-Puig, P.; Gryfe, R.; Shepherd, L.E.; et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2003, 349, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Carethers, J.M.; Smith, E.J.; Behling, C.A.; Nguyen, L.; Tajima, A.; Doctolero, R.T.; Cabrera, B.L.; Goel, A.; Arnold, C.A.; Miyai, K.; et al. Use of 5-fluorouracil and survival in patients with microsatelliteunstable colorectal cancer. Gastroenterology 2004, 126, 394–401. [Google Scholar] [CrossRef]
- Jover, R.; Zapater, P.; Castells, A.; Llor, X.; Andreu, M.; Cubiella, J.; Piñol, V.; Xicola, R.M.; Bujanda, L.; Reñé, J.M.; et al. Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut 2006, 55, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A.; Foster, N.R.; Thibodeau, S.N.; Marsoni, S.; Monges, G.; Labianca, R.; Kim, G.P.; Yothers, G.; Allegra, C.; Moore, M.J.; et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J. Natl. Cancer Inst. 2011, 103, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Vilar, E.; Gruber, S.B. Microsatellite instability in colorectal cancer-the stable evidence. Nat. Rev. Clin. Oncol. 2010, 7, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Webber, E.M.; Kauffman, T.L.; O’Connor, E.; Goddard, K.A. Systematic review of the predictive effect of MSI status in colorectal cancer patients undergoing 5FU-based chemotherapy. BMC Cancer 2015, 15, 156. [Google Scholar] [CrossRef] [PubMed]
- Des Guetz, G.; Schischmanoff, O.; Nicolas, P.; Perret, G.Y.; Morere, J.F.; Uzzan, B. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur. J. Cancer 2009, 45, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Des Guetz, G.; Uzzan, B.; Nicolas, P.; Schischmanoff, O.; Perret, G.Y.; Morere, J.F. Microsatellite instability does not predict the efficacy of chemotherapy in metastatic colorectal cancer. A systematic review and meta-analysis. Anticancer Res. 2009, 29, 1615–1620. [Google Scholar] [PubMed]
| Study, Year | Number of Included Trials | Number of Patients | CRC Stage | Received Treatment | MSI Status | Main Findings | Predictive Value of MSI Status |
|---|---|---|---|---|---|---|---|
| Popat et al., 2005 [53] | 32 | 7642 | I–IV | 5-FU-based adjuvant chemotherapy vs. control group | 1277 MSI | MSI-H status established as a prognostic factor; no benefit from adjuvant FU for MSI patients | Not assessed |
| Guastadisegni et al., 2010 [64] | 31 | 12,782 | I–IV | 5-FU-based adjuvant chemotherapy in combination with levamisole or leucovorin (in 6 studies) or mitomycin (in 1 study) | 14% MSI (396 MSI, 2467 MSS) | MSI-H status established as a prognostic factor (association between MSI and favourable prognosis in term of OS and DFS; inconclusive results about predictive value of MSI status due to the high inter-study heterogeneity | Inconclusive results |
| Webber et al., 2015 [80] | 16 | 9312 | I–IV | 5-FU-based chemotherapy vs. control group | 15% MSI | No difference in the effect of treatment based on MSI status | Not proven |
| Des Guetz et al., 2009 [81] | 7 | 3690 | II–III | 5-FU-based adjuvant chemotherapy vs. control group | 14% MSI (454 MSI-H; 3690 MSS) | MSI-H status established as a predictive factor of non response to 5-FU-based chemotherapy in CRC patients stage II or III | Proven for patients stage II/III |
| Des Guetz et al., 2009 [82] | 6 | 964 | IV | 5-FU-based chemotherapy or combinations of 5-FU or capecitabine with oxaliplatin and/or irinotecan | 9% MSI (91 MSI-H, 873 MSS) | No difference in the effect of treatment of patients with metastatic CRC in terms of RR based on MSI status | Not proven for mCRC patients |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Copija, A.; Waniczek, D.; Witkoś, A.; Walkiewicz, K.; Nowakowska-Zajdel, E. Clinical Significance and Prognostic Relevance of Microsatellite Instability in Sporadic Colorectal Cancer Patients. Int. J. Mol. Sci. 2017, 18, 107. https://doi.org/10.3390/ijms18010107
Copija A, Waniczek D, Witkoś A, Walkiewicz K, Nowakowska-Zajdel E. Clinical Significance and Prognostic Relevance of Microsatellite Instability in Sporadic Colorectal Cancer Patients. International Journal of Molecular Sciences. 2017; 18(1):107. https://doi.org/10.3390/ijms18010107
Chicago/Turabian StyleCopija, Angelika, Dariusz Waniczek, Andrzej Witkoś, Katarzyna Walkiewicz, and Ewa Nowakowska-Zajdel. 2017. "Clinical Significance and Prognostic Relevance of Microsatellite Instability in Sporadic Colorectal Cancer Patients" International Journal of Molecular Sciences 18, no. 1: 107. https://doi.org/10.3390/ijms18010107
APA StyleCopija, A., Waniczek, D., Witkoś, A., Walkiewicz, K., & Nowakowska-Zajdel, E. (2017). Clinical Significance and Prognostic Relevance of Microsatellite Instability in Sporadic Colorectal Cancer Patients. International Journal of Molecular Sciences, 18(1), 107. https://doi.org/10.3390/ijms18010107