Multifunctional Composite Microcapsules for Oral Delivery of Insulin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of the Particles
2.2. In Vitro Release Kinetic Experiment
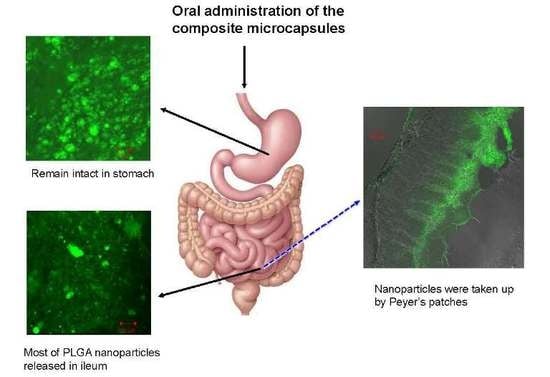
2.3. Studies of Disposition of the Particles in the Gastrointestinal Tract and Absorption Mechanism in Ileum
2.4. Biological Activity of Insulin
2.5. Hypoglycemic Effect of the Multifunctional Composite Microcapsules
3. Materials and Methods
3.1. Materials and Animals
3.2. Preparation of Insulin-Sodium Deoxycholate Complex (Ins-SD-Comp) and the PLGA NPs
3.3. Enzymatic Degradation Studies
3.4. Preparation of Multifunctional Composite Microcapsules
3.5. Characterization of the Particles
3.6. In Vitro Release Kinetic Experiment
3.7. Biological Activity of Insulin
3.8. Studies of Disposition of the Particles and Absorption Mechanism in the Gastrointestinal Tract
3.9. In Vivo Evaluation on Diabetic Rats
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pillai, O.; Panchagnula, R. Insulin therapies—Past, present and future. Drug Discov. Today 2001, 6, 1056–1061. [Google Scholar] [CrossRef]
- Viehof, A.; Javot, L.; Béduneau, A.; Pellequer, Y.; Lamprecht, A. Oral insulin delivery in rats by nanoparticles prepared with non-toxic solvents. Int. J. Pharm. 2013, 443, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Mishra, R.; Rana, D.; Kundu, P.P. Strategies for effective oral insulin delivery with modified chitosan nanoparticles: A review. Prog. Polym. Sci. 2012, 37, 1457–1475. [Google Scholar] [CrossRef]
- Arbit, E. The physiological rationale for oral insulin administration. Diabetes Technol. Ther. 2004, 6, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Arbit, E.; Kidron, M. Oral insulin: The rationale for this approach and current developments. J. Diabetes Sci. Technol. 2009, 3, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.; Ganguly, K.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric hydrogels for oral insulin delivery. J. Control. Release 2013, 165, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Damgé, C.; Maincent, P.; Ubrich, N. Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats. J. Control. Release 2007, 117, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Zinman, B.; Groenewoud, Y.; Vranic, M.; Giacca, A. Hepatic glucose production is regulated both by direct hepatic and extrahepatic effects of insulin in humans. Diabetes 1996, 45, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Sonaje, K.; Lin, K.J.; Wey, S.P.; Lin, C.K.; Yeh, T.H.; Nguyen, H.N.; Hsu, C.W.; Yen, T.C.; Juang, J.H.; Sung, H.W. Biodistribution, pharmacodynamics and pharmacokinetics of insulin analogues in a rat model: Oral delivery using pH-Responsive nanoparticles vs. subcutaneous injection. Biomaterials 2010, 31, 6849–6858. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, R.; Ahmad, Z.; Soric, M.; Stride, E.; Edirisinghe, M. Nanoparticle delivery systems formed using electrically sprayed co-flowing excipients and active agent. J. Biomed. Nanotechnol. 2011, 7, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liang, N.; Kawashima, Y.; Xia, D.; Cui, F. Hydrophobic ion pairing of an insulin-sodium deoxycholate complex for oral delivery of insulin. Int. J. Nanomed. 2011, 6, 3049–3056. [Google Scholar]
- Sun, S.; Liang, N.; Piao, H.; Yamamoto, H.; Kawashima, Y.; Cui, F. Insulin-S.O (sodium oleate) complex-loaded PLGA nanoparticles: Formulation, characterization and in vivo evaluation. J. Microencapsul. 2010, 27, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Ekemen, Z.; Chang, H.; Ahmad, Z.; Bayram, C.; Rong, Z.; Denkbas, E.B.; Stride, E.; Vadgama, P.; Edirisinghe, M. Fabrication of biomaterials via controlled protein bubble generation and manipulation. Biomacromolecules 2011, 12, 4291–4300. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Haj-Ahmad, R.; Rasekh, M.; Arshad, M.S.; Smith, A.; van der Merwe, S.M.; Li, X.; Chang, M.-W.; Ahmad, Z. Pharmaceutical and biomaterial engineering via electrohydrodynamic atomization technologies. Drug Discov. Today 2016. [Google Scholar] [CrossRef] [PubMed]
- Sonaje, K.; Chen, Y.J.; Chen, H.L.; Wey, S.P.; Juang, J.H.; Nguyen, H.N.; Hsu, C.W.; Lin, K.J.; Sung, H.W. Enteric-coated capsules filled with freeze-dried chitosan/poly(γ-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials 2010, 31, 3384–3394. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, Y.; Liu, C.S.; Chen, Q.; Wang, G.H.; Guo, W.; Wu, X.E.; Li, D.H.; Wu, W.D.; Chen, X.D. Enteric-coated capsules filled with mono-disperse micro-particles containing PLGA-lipid-PEG nanoparticles for oral delivery of insulin. Int. J. Pharm. 2015, 484, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.M.; Zhou, L.; Guo, X.D.; Jiang, W.; Ling, L.; Qian, Y.; Luo, K.Q.; Zhang, L.J. HP55-coated capsule containing PLGA/RS nanoparticles for oral delivery of insulin. Int. J. Pharm. 2012, 425, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.P.; Ribeiro, A.J.; Houng, S.; Veiga, F.; Neufeld, R.J. Nanoparticulate delivery system for insulin: Design, characterization and in vitro/in vivo bioactivity. Eur. J. Pharm. Sci. 2007, 30, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liang, N.; Yamamoto, H.; Kawashima, Y.; Cui, F.; Yan, P. pH-sensitive poly(lactide-co-glycolide) nanoparticle composite microcapsules for oral delivery of insulin. Int. J. Nanomed. 2015, 10, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shan, C.; Zu, Y.; Zhang, Y.; Wang, W.; Wang, K.; Sui, X.; Li, R. Preparation, characterization, and evaluation in vivo of Ins-SiO2-HP55 (insulin-loaded silica coating HP55) for oral delivery of insulin. Int. J. Pharm. 2013, 454, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Edgar, K. Cellulose esters in drug delivery. Cellulose 2007, 14, 49–64. [Google Scholar] [CrossRef]
- Alibolandi, M.; Alabdollah, F.; Sadeghi, F.; Mohammadi, M.; Abnous, K.; Ramezani, M.; Hadizadeh, F. Dextran-b-poly(lactide-co-glycolide) polymersome for oral delivery of insulin: In vitro and in vivo evaluation. J. Control. Release 2016, 227, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Eaimtrakarn, S.; Rama Prasad, Y.V.; Ohno, T.; Konishi, T.; Yoshikawa, Y.; Shibata, N.; Takada, K. Absorption enhancing effect of Labrasol on the intestinal absorption of insulin in rats. J. Drug Target. 2002, 10, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.P.; Alonso, M.J.; Csaba, N. Polymer-based oral peptide nanomedicines. Ther. Deliv. 2012, 3, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Deng, Y.J. Oil-based formulations for oral delivery of insulin. J. Pharm. Pharmacol. 2004, 56, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, T.; Kimura, Y.; Artursson, P. Absorption enhancement through intracellular regulation of tight junction permeability by medium chain fatty acids in Caco-2 cells. J. Pharmacol. Exp. Ther. 1998, 284, 362–369. [Google Scholar] [PubMed]
- Sakai, M.; Imai, T.; Ohtake, H.; Azuma, H.; Otagiri, M. Effects of absorption enhancers on the transport of model compounds in Caco-2 cell monolayers: Assessment by confocal laser scanning microscopy. J. Pharm Sci. 1997, 86, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Bahar, R.J.; Stolz, A. Bile acid transport. Gastroenterol. Clin. N. Am. 1999, 28, 27–58. [Google Scholar] [CrossRef]
- Ferrebee, C.B.; Dawson, P.A. Metabolic effects of intestinal absorption and enterohepatic cycling of bile acids. Acta Pharm. Sin. B 2015, 5, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Shrestha, N.; Correia, A.; Shahbazi, M.-A.; Sarmento, B.; Hirvonen, J.; Veiga, F.; Seiça, R.; Ribeiro, A.; Santos, H.A. Dual chitosan/albumin-coated alginate/dextran sulfate nanoparticles for enhanced oral delivery of insulin. J. Control. Release 2016, 232, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Takenaga, M.; Yamaguchi, Y.; Kitagawa, A.; Ogawa, Y.; Kawai, S.; Mizushima, Y.; Igarashi, R. Optimum formulation for sustained-release insulin. Int. J. Pharm. 2004, 271, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, C.; Goldfine, I.D.; Whittaker, J. The metabolic and mitogenic effects of both insulin and insulin-like growth factor are enhanced by transfection of insulin receptors into NIH3T3 fibroblasts. J. Biol. Chem. 1989, 264, 8606–8611. [Google Scholar] [PubMed]
- Wu, Z.M.; Ling, L.; Zhou, L.Y.; Guo, X.D.; Jiang, W.; Qian, Y.; Luo, K.Q.; Zhang, L.J. Novel preparation of PLGA/HP55 nanoparticles for oral insulin delivery. Nanoscale Res. Lett. 2012, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Araújo, F.; Silva, C.; Pereira, C.; Reis, S.; Santos, H.A.; Sarmento, B. Polymer-based nanoparticles for oral insulin delivery: Revisited approaches. Biotechnol. Adv. 2015, 33, 1342–1354. [Google Scholar] [CrossRef] [PubMed]
- Pridgen, E.M.; Alexis, F.; Farokhzad, O.C. Polymeric nanoparticle technologies for oral drug delivery. Clin. Gastroenterol. Hepatol. 2014, 12, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, T.F.; Lenourry, A.; Charrueau, C.; Chaumeil, J.C. The use of polysaccharides to target drugs to the colon. Carbohydr. Polym. 2002, 48, 219–231. [Google Scholar] [CrossRef]
- Damgé, C.; Michel, C.; Aprahamian, M.; Couvreur, P. New approach for oral administration of insulin with polyalkylcyanoacrylate nanocapsules as drug carrier. Diabetes 1988, 37, 246–251. [Google Scholar] [CrossRef] [PubMed]










© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Liang, N.; Gong, X.; An, W.; Kawashima, Y.; Cui, F.; Yan, P. Multifunctional Composite Microcapsules for Oral Delivery of Insulin. Int. J. Mol. Sci. 2017, 18, 54. https://doi.org/10.3390/ijms18010054
Sun S, Liang N, Gong X, An W, Kawashima Y, Cui F, Yan P. Multifunctional Composite Microcapsules for Oral Delivery of Insulin. International Journal of Molecular Sciences. 2017; 18(1):54. https://doi.org/10.3390/ijms18010054
Chicago/Turabian StyleSun, Shaoping, Na Liang, Xianfeng Gong, Weiwei An, Yoshiaki Kawashima, Fude Cui, and Pengfei Yan. 2017. "Multifunctional Composite Microcapsules for Oral Delivery of Insulin" International Journal of Molecular Sciences 18, no. 1: 54. https://doi.org/10.3390/ijms18010054
APA StyleSun, S., Liang, N., Gong, X., An, W., Kawashima, Y., Cui, F., & Yan, P. (2017). Multifunctional Composite Microcapsules for Oral Delivery of Insulin. International Journal of Molecular Sciences, 18(1), 54. https://doi.org/10.3390/ijms18010054