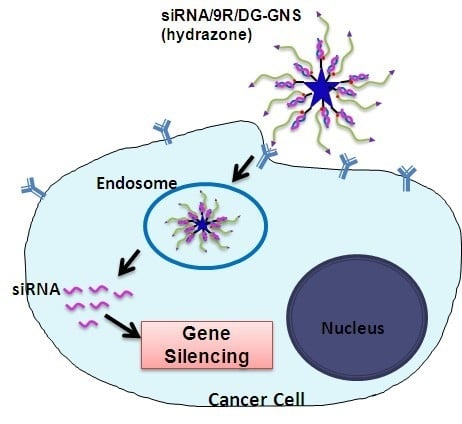
Targeted Delivery of siRNA with pH-Responsive Hybrid Gold Nanostars for Cancer Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of siRNA/Gold Nanostars (GNS) Complex
2.1.1. The Influence of Reaction Conditions on the Growth of GNS
2.1.2. Size Distribution, Zeta Potential, Morphology and Optical Characterization of the GNS Complex


2.1.3. Analysis of Binding Ability of GNS Complex and siRNA

2.2. Cytotoxicity of GNS Complex

2.3. In Vitro Cell Uptake

2.4. Gene Silencing Efficiency of siRNA/GNS Complex

2.5. Effects of siRNA/GNS Complex on Cancer Cell Growth

3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of Ligands LA-Lys-9R, LA-Lys-9R/PEG-DG, and LA-Lys-9R/PEG-DG (Hydrazone)
3.2.2. Preparation and Characterization of GNS
Synthesis of Gold Seeds
Synthesis of GNS
Characterization of GNS
3.2.3. Preparation and Characterization of Hybrid GNS Complex
3.2.4. Preparation and Characterization of the Mixture of siRNA and GNS Complex
3.2.5. Cell Experiments
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assays
In Vitro Cellular Uptake
Western Blot Analysis
CCK-8 (Cell Counting Kit-8) Method to Analyze the Effect of siRNA/GNS Complex on Cancer Cells Growth
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GNS | Gold nanostar |
| COX-2 | Cyclooxygenase-2 |
| siRNA | Small interfering RNA |
| siCOX-2 | COX-2 siRNA |
| DG | 2-Amino-2-deoxy-d-glucose |
| PEG | Polyethylene glycol |
| 9R | 9-d-arginine |
| LA | α-Lipoic acid |
| GLUT1 | Glucose transporter 1 |
| TEM | Transmission electron microscopy |
| AA | Ascorbic acid |
| DLS | Dynamic light scattering |
| PDI | Polydispersity index |
| HUVEC | Human umbilical vein endothelial cells |
| HepG2 | Human hepatocellular liver carcinoma |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| CCK-8 | Cell counting kit-8 |
| Lys | Lysine |
| UV-VIS-NIR | Ultraviolet-visible-near infrared |
| siNC | Negative control siRNA |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electropheresis |
References
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2010, 29, 781. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.K.; Sung, J.J.Y.; Lee, C.W.; Yu, J.; Cho, C.H. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: An update on the molecular mechanisms. Cancer Lett. 2010, 295, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Khan, N.; Tiwari, R.P.; Sah, N.K.; Prasad, G.B.; Bisen, P.S. Biology of Cox-2: An application in cancer therapeutics. Curr. Drug Targets 2011, 12, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Chaki, R.; Mandal, V.; Mandal, S.C. COX-2 as a target for cancer chemotherapy. Pharmacol. Rep. 2010, 62, 233–244. [Google Scholar] [CrossRef]
- Xu, L.; Stevens, J.; Hilton, M.B.; Seaman, S.; Conrads, T.P.; Veenstra, T.D.; Logsdon, D.; Morris, H.; Swing, D.A.; Patel, N.L. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci. Transl. Med. 2014, 6, 242ra84. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Sung, M.; Park, J.; Yang, H.; Kim, M.; Hur, H.; Hwang, J.-T. Poly-γ-Glutamic Acid Induces Apoptosis via Reduction of COX-2 Expression in TPA-Induced HT-29 Human Colorectal Cancer Cells. Int. J. Mol. Sci. 2015, 16, 7577–7586. [Google Scholar] [CrossRef] [PubMed]
- Jarupongprapa, S.; Ussavasodhi, P.; Katchamart, W. Comparison of gastrointestinal adverse effects between cyclooxygenase-2 inhibitors and non-selective, non-steroidal anti-inflammatory drugs plus proton pump inhibitors: A systematic review and meta-analysis. J. Gastroenterol. 2013, 48, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Maund, E.; McDaid, C.; Rice, S.; Wright, K.; Jenkins, B.; Woolacott, N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: A systematic review. Br. J. Anaesth. 2011, 106, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.E.; Davis, M.E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 2015, 14, 843. [Google Scholar] [CrossRef] [PubMed]
- Ozpolat, B.; Sood, A.K.; Lopez-Berestein, G. Liposomal siRNA nanocarriers for cancer therapy. Adv. Drug Deliv. Rev. 2014, 66, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Wittrup, A.; Lieberman, J. Knocking down disease: A progress report on siRNA therapeutics. Nat. Rev. Genet. 2015, 16, 543. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Kim, H.J.; Mi, P.; Zheng, M.; Takemoto, H.; Toh, K.; Kim, B.S.; Hayashi, K.; Naito, M.; Matsumoto, Y. Targeted systemic delivery of siRNA to cervical cancer model using cyclic RGD-installed unimer polyion complex-assembled gold nanoparticles. J. Control. Release 2016, 244, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Takemoto, H.; Yi, Y.; Zheng, M.; Maeda, Y.; Chaya, H.; Hayashi, K.; Mi, P.; Pittella, F.; Christie, R.J. Precise engineering of siRNA delivery vehicles to tumors using polyion complexes and gold nanoparticles. ACS Nano 2014, 8, 8979–8991. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.J.; Tzeng, S.Y.; Green, J.J. Degradable polymer-coated gold nanoparticles for co-delivery of DNA and siRNA. Acta Biomater. 2015, 11, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-K.; Yu, X.-F.; Wang, J.-H.; Li, Z.-B.; Li, P.-H.; Wang, H.; Song, L.; Chu, P.K.; Li, C. Gold-nanorods-siRNA nanoplex for improved photothermal therapy by gene silencing. Biomaterials 2016, 78, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Yang, C.; Wang, Q.; Zeng, S.; Hu, R.; Lin, G.; Tian, J.; Hu, S.; Lan, R.F.; Yoon, H.S. A light-driven therapy of pancreatic adenocarcinoma using gold nanorods-based nanocarriers for co-delivery of doxorubicin and siRNA. Theranostics 2015, 5, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Hwang, H.-J.; Shin, S.W.; Choi, J.-W.; Um, S.H.; Oh, B.-K. A novel albumin nanocomplex containing both small interfering RNA and gold nanorods for synergetic anticancer therapy. Nanoscale 2015, 7, 9229–9237. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, H.; Galbraith, K.K.; Kurisu, J.; Imahori, H.; Murakami, T.; Kengaku, M. Surface chemistry for cytosolic gene delivery and photothermal transgene expression by gold nanorods. Sci. Rep. 2017, 7, 4694. [Google Scholar] [CrossRef] [PubMed]
- Sardo, C.; Bassi, B.; Craparo, E.F.; Scialabba, C.; Cabrini, E.; Dacarro, G.; D’Agostino, A.; Taglietti, A.; Giammona, G.; Pallavicini, P. Gold nanostar—Polymer hybrids for siRNA delivery: Polymer design towards colloidal stability and in vitro studies on breast cancer cells. Int. J. Pharm. 2017, 519, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Chen, J.; Hu, Y.; Li, X.; Wang, H.; Shen, M.; Shi, X. Dendrimer-Stabilized Gold Nanostars as a Multifunctional Theranostic Nanoplatform for CT Imaging, Photothermal Therapy, and Gene Silencing of Tumors. Adv. Healthc. Mater. 2016, 5, 3203–3213. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G. Single continuous wave laser induced photodynamic/plasmonic photothermal therapy using photosensitizer-functionalized gold nanostars. Adv. Mater. 2013, 25, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Khoury, C.G.; Wilson, C.M.; Grant, G.A.; Bennett, A.J.; Vo-Dinh, T. In vivo particle tracking and photothermal ablation using plasmon-resonant gold nanostars. Nanomedicine 2012, 8, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Ai, K.; Ozaki, Y. Environmentally friendly synthesis of highly monodisperse biocompatible gold nanoparticles with urchin-like shape. Langmuir 2008, 24, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Fales, A.M.; Vo-Dinh, T. TAT peptide-functionalized gold nanostars: Enhanced intracellular delivery and efficient NIR photothermal therapy using ultralow irradiance. J. Am. Chem. Soc. 2012, 134, 11358–11361. [Google Scholar] [CrossRef] [PubMed]
- Nergiz, S.Z.; Gandra, N.; Tadepalli, S.; Singamaneni, S. Multifunctional hybrid nanopatches of graphene oxide and gold nanostars for ultraefficient photothermal cancer therapy. ACS Appl. Mater. Interfaces 2014, 6, 16395–16402. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 2000, 60, 4440–4445. [Google Scholar] [PubMed]
- Hediger, M.A.; Rhoads, D.B. Molecular physiology of sodium-glucose cotransporters. Physiol. Rev. 1994, 74, 993–1026. [Google Scholar] [PubMed]
- Amann, T.; Maegdefrau, U.; Hartmann, A.; Agaimy, A.; Marienhagen, J.; Weiss, T.S.; Stoeltzing, O.; Warnecke, C.; Schölmerich, J.; Oefner, P.J. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am. J. Pathol. 2009, 174, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Wellberg, E.A.; Johnson, S.; Finlay-Schultz, J.; Lewis, A.S.; Terrell, K.L.; Sartorius, C.A.; Abel, E.D.; Muller, W.J.; Anderson, S.M. The glucose transporter GLUT1 is required for ErbB2-induced mammary tumorigenesis. Breast Cancer Res. 2016, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Simons, A.L.; Ahmad, I.M.; Mattson, D.M.; Dornfeld, K.J.; Spitz, D.R. 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007, 67, 3364–3370. [Google Scholar] [CrossRef] [PubMed]
- Simons, A.L.; Fath, M.A.; Mattson, D.M.; Smith, B.J.; Walsh, S.A.; Graham, M.M.; Hichwa, R.D.; Buatti, J.M.; Dornfeld, K.; Spitz, D.R. Enhanced response of human head and neck cancer xenograft tumors to cisplatin combined with 2-deoxy-d-glucose correlates with increased 18 F-FDG uptake as determined by PET imaging. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Maschek, G.; Savaraj, N.; Priebe, W.; Braunschweiger, P.; Hamilton, K.; Tidmarsh, G.F.; De Young, L.R.; Lampidis, T.J. 2-deoxy-d-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004, 64, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Takahashi, N.; Tanno, S.; Nagamine, M.; Takakusaki, K.; Okumura, T. A selective orexin-1 receptor antagonist, SB334867, blocks 2-DG-induced gastric acid secretion in rats. Neurosci. Lett. 2005, 376, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zielonka, J.; Dranka, B.P.; McAllister, D.; Mackinnon, A.C.; Joseph, J.; Kalyanaraman, B. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 2012, 72, 2634–2644. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold nanostars: Surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 2012, 23, 075102. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Yang, C.; Zhu, J.-J. Fabrication of gold nanorods with tunable longitudinal surface plasmon resonance peaks by reductive dopamine. Langmuir 2015, 31, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Liu, M.; Wang, R.F.; Yan, P.; Zhang, C.L.; Ma, C.; Zhao, Q.; Yin, L.; Zhao, G.Y.; Guo, F.Q. Noninvasive imaging of c (RGD) 2–9R as a potential delivery carrier for transfection of siRNA in malignant tumors. J. Labelled Comp. Radiopharm. 2017, 6, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Huang, W.; Kang, L.; Jin, M.; Fan, B.; Jin, H.; Wang, Q.-M.; Gao, Z. siRNA-loaded poly (histidine-arginine) 6-modified chitosan nanoparticle with enhanced cell-penetrating and endosomal escape capacities for suppressing breast tumor metastasis. Int. J. Nanomed. 2017, 12, 3221–3234. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Honen, R.; Turjeman, K.; Gabizon, A.; Kost, J.; Barenholz, Y. Ultrasound triggered release of cisplatin from liposomes in murine tumors. J. Control. Release 2009, 137, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, T.; Wu, X.; Li, L.; Tan, L.; Chen, D.; Tang, F. Targeting gold nanoshells on silica nanorattles: A drug cocktail to fight breast tumors via a single irradiation with near-infrared laser light. Adv. Mater. 2012, 24, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Kam, N.W.S.; Liu, Z.; Dai, H. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J. Am. Chem. Soc. 2005, 127, 12492–12493. [Google Scholar] [CrossRef] [PubMed]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Kuwano, K.; Ochiya, T. Development of Small RNA Delivery Systems for Lung Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 5254–5270. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, S.; Ling, Y.; Meng, G.; Yang, Y.; Zhang, W. pH-responsive hybrid quantum dots for targeting hypoxic tumor siRNA delivery. J. Control. Release 2015, 220, 529–544. [Google Scholar] [CrossRef] [PubMed]
| Samples | Hydrodynamic Diameter (nm) | Polydispersity | Zeta Potential (mV) |
|---|---|---|---|
| GNS | 72.5 ± 3.2 | 0.303 ± 0.024 | 2.42 ± 0.22 |
| 9R-GNS | 77.8 ± 9.7 | 0.406 ± 0.020 | 15.53 ± 2.04 |
| 9R/DG-GNS | 230.7 ± 8.6 | 0.251 ± 0.013 | 11.26 ± 3.21 |
| 9R/DG-GNS (hydrazone) | 210.5 ± 10.3 | 0.231 ± 0.030 | 12.35 ± 2.28 |
| Type of siRNA | Sense (5′-3′) | Antisense (5′-3′) |
|---|---|---|
| COX-2 siRNA | AACUGCUCAACACCGGAA | AUUCCGGUGUUGAGCAGU |
| NC siRNA | UUCUCCGAACGUGUCACGU | ACGUGACACGUUCGGAGA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Liu, W.; Cheng, Z.; Yao, K.; Yang, Y.; Xu, B.; Su, G. Targeted Delivery of siRNA with pH-Responsive Hybrid Gold Nanostars for Cancer Treatment. Int. J. Mol. Sci. 2017, 18, 2029. https://doi.org/10.3390/ijms18102029
Zhu H, Liu W, Cheng Z, Yao K, Yang Y, Xu B, Su G. Targeted Delivery of siRNA with pH-Responsive Hybrid Gold Nanostars for Cancer Treatment. International Journal of Molecular Sciences. 2017; 18(10):2029. https://doi.org/10.3390/ijms18102029
Chicago/Turabian StyleZhu, Hongyan, Wanwan Liu, Ziting Cheng, Ke Yao, Yu Yang, Bohui Xu, and Gaoxing Su. 2017. "Targeted Delivery of siRNA with pH-Responsive Hybrid Gold Nanostars for Cancer Treatment" International Journal of Molecular Sciences 18, no. 10: 2029. https://doi.org/10.3390/ijms18102029
APA StyleZhu, H., Liu, W., Cheng, Z., Yao, K., Yang, Y., Xu, B., & Su, G. (2017). Targeted Delivery of siRNA with pH-Responsive Hybrid Gold Nanostars for Cancer Treatment. International Journal of Molecular Sciences, 18(10), 2029. https://doi.org/10.3390/ijms18102029