Effects of Extremely Low Frequency Electromagnetic Fields on Melanogenesis through p-ERK and p-SAPK/JNK Pathways in Human Melanocytes
Abstract
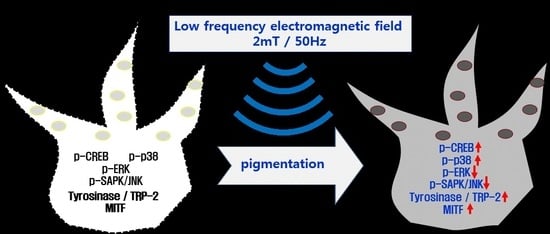
:1. Introduction
2. Result
2.1. Cell Proliferation and Mitochondrial Activity
2.2. Cytotoxicity
2.3. Melanin Content
2.4. Tyrosinase Activity
2.5. RT-qPCR
2.6. Western Blotting
2.7. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Exposure to Extremely Low Frequency Electromagnetic Fields (ELF-EMFs)
4.3. Cell Proliferation Measurement and Mitochondrial Activity Assay
4.4. Cytotoxicity Assay
4.5. Melanin Content Assay
4.6. Tyrosinase Activity Assay
4.7. RT-qPCR
4.8. Western Blotting
4.9. Immunohistochemistry
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. Anais Bras Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin Releasing Hormone and Proopiomelanocortin Involvement in the Cutaneous Response to Stress. Physiol. Rev. 2000, 80, 979–1010. [Google Scholar] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Zbytek, B.; Tobin, D.J.; Theoharides, T.C.; Rivier, J. Key role of CRF in the skin stress response system. Endocr. Rev. 2013, 34, 827–884. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2011, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Plonka, P.M.; Schallreuter, K.U.; Paus, R.; Tobin, D.J. Hair follicle pigmentation. J. Investig. Dermatol. 2005, 124, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Endou, M.; Aoki, H. Prevention of hair graying by factors that promote the growth and differentiation of melanocytes. J. Dermatol. 2014, 41, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.K.; Majumder, P.P.; Nordlund, J.J. Genetic epidemiology of vitiligo: Multilocus recessivity cross-validated. Am. J. Hum. Genet. 1994, 55, 981–990. [Google Scholar] [PubMed]
- Halder, R.M.; Chappell, J.L. Vitiligo update. Semin. Cutan. Med. Surg. 2009, 28, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Namazi, M.R. Neurogenic dysregulation, oxidative stress, autoimmunity, and melanocytorrhagy in vitiligo: Can they be interconnected? Pigment Cell Melanoma Res. 2007, 20, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Yazdani Abyaneh, M.; Griffith, R.D. Narrowband ultraviolet B phototherapy in combination with other therapies for vitiligo: Mechanisms and efficacies. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Gupta, A. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar] [PubMed]
- Lan, C.C.; Wu, C.S.; Chiou, M.H.; Chiang, T.Y.; Yu, H.S. Low-energy helium-neon laser induces melanocyte proliferation via interaction with type IV collagen: Visible light as a therapeutic option for vitiligo. Br. J. Dermatol. 2009, 161, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Lacy-Hulbert, A.; Metcalfe, J.C.; Hesketh, R. Biological responses to electromagnetic fields. FASEB J. 1998, 12, 395–420. [Google Scholar] [PubMed]
- Tsai, M.T.; Li, W.J.; Tuan, R.S.; Chang, W.H. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J. Orthop. Res. 2009, 27, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Hsieh, D.K.; Lin, P.C.; Chiu, H.T.; Chiou, T.W. Pulsed electromagnetic fields accelerate proliferation and osteogenic gene expression in human bone marrow mesenchymal stem cells during osteogenic differentiation. Bioelectromagnetics 2010, 31, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Sert, C.; Mustafa, D.; Düz, M.Z.; Akşen, F.; Kaya, A. The preventive effect on bone loss of 50-Hz, 1-mT electromagnetic field in ovariectomized rats. J. Bone Mine Metab. 2002, 20, 345–349. [Google Scholar] [CrossRef] [PubMed]
- McLeod, K.J.; Rubin, C.T. The effect of low-frequency electrical fields on osteogenesis. J. Bone Jt. Surg. 1992, 74, 920–929. [Google Scholar] [CrossRef]
- Piacentini, R.; Ripoli, C.; Mezzogori, D.; Azzena, G.B.; Grassi, C. Extremely low frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Ca(v)1-channel activity. J. Cell Physiol. 2008, 215, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Cuccurazzu, B.; Leone, L.; Podda, M.V.; Piacentini, R.; Riccardi, E.; Ripoli, C.; Azzena, G.B.; Grassi, C. Exposure to extremely low-frequency (50 Hz) electromagnetic fields enhances adult hippocampal neurogenesis in C57BL/6 mice. Exp. Neurol. 2010, 226, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Seo, Y.K.; Yoon, H.H.; Kim, S.C.; Kim, S.M.; Song, K.Y.; Park, J.K. Neural stimulation on human bone marrow-derived mesenchymal stem cells by extremely low frequency electromagnetic fields. Biotechnol. Prog. 2012, 28, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Paus, R.; Schadendorf, D. melanocyte as “sensory” and regulatory cells in the epidermis. J. Theor. Biol. 1993, 164, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.B.; Santone, K.S.; Acosta, D. Evaluation of cyto¬toxicity in cultured cells by enzyme leakage. Methods Cell Sci. 1980, 6, 113–116. [Google Scholar]
- Naarala, J.; Hoyto, A.; Markkanen, A. Cellular effects of electromagnetic fields. Altern. Lab. Anim. 2004, 32, 355–360. [Google Scholar] [PubMed]
- Seeliger, C.; Falldorf, K.; Sachtleben, J.; van Griensven, M. Low-frequency pulsed electromagnetic fields significantly improve time of closure and proliferation of human tendon fibroblasts. Eur. J. Med. Res. 2014, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Leone, L.; Podda, M.V.; Grassi, C. Impact of electromagnetic fields on stem cells: Common mechanisms at the crossroad between adult neurogenesis and osteogenesis. Front. Cell Neurosci. 2015, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Costantini, E.; Kamal, M.A.; Reale, M. Experimental model for ELF-EMF exposure: Concern for human health. Saudi J. Biol. Sci. 2015, 22, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Botchkareva, N.V.; Khlgatian, M.; Longley, B.J.; Botchkarev, V.A.; Gilchrest, B.A. SCF/c-kit signaling is required for cyclic regeneration of the hair pigmentation unit. FASEB J. 2001, 15, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Winder, A.; Kobayashi, T.; Tsukamoto, K.; Urabe, K.; Aroca, P.; Kameyama, K.; Hearing, V.J. The tyrosinase gene family: Interactions of melanogenic proteins to regulate melanogenesis. Cell. Mol. Biol. Res. 1994, 40, 613–626. [Google Scholar] [PubMed]
- Hemesath, T.J.; Price, E.R.; Takemoto, C.; Badalian, T.; Fisher, D.E. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature 1998, 391, 298–301. [Google Scholar] [PubMed]
- Abdel-Malek, Z.; Swope, V.B.; Suzuki, I.; Akcali, C.; Harriger, M.D.; Boyce, S.T.; Urabe, K.; Hearing, V.J. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc. Natl. Acad. Sci. USA 1995, 92, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.; Todd, C.; Cresswell, J.E.; Thody, A.J. Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7 alpha-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J. Cell Sci. 1994, 107, 205–211. [Google Scholar] [PubMed]
- Suzuki, I.; Cone, R.D.; Im, S.; Nordlund, J.; Abdel-Malek, Z.A. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology 1996, 137, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Jiang, M.; Xu, X.; Guan, M.; Wu, J.; Chen, Q.; Xiang, L. The effects of NB-UVB on the hair follicle-derived neural crest stem cells differentiating into melanocyte lineage in vitro. J. Dermatol. Sci. 2012, 66, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Hu, Y.; He, L. Regulation of melanocyte pivotal transcription factor MITF by some other transcription factors. Mol. Cell Biochem. 2011, 354, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.J. Aromatic-turmerone inhibits α-MSH and IBMX-induced melanogenesis by inactivating CREB and MITF signaling pathways. Arch. Dermatol. Res. 2011, 303, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhang, B.X.; Zhang, C.; Shen, N.; Zhang, Y.Y.; Wang, A.X.; Tu, C.X. Ginsenosides Rb1 and Rg1 Stimulate Melanogenesis in Human Epidermal Melanocytes via PKA/CREB/MITF Signaling. Evid. Based Complement. Altern. Med. 2014, 2014, 892073. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Balcos, M.C.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Kim, M.K.; Kim, D.S. ERK Activation by Fucoidan Leads to Inhibition of Melanogenesis in Mel-Ab Cells. Korean J. Physiol. Pharmacol. 2015, 19, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Jeon, H.B.; Lim, H.; Shin, J.H.; Park, S.J.; Jo, Y.K.; Oh, W.; Yang, Y.S.; Cho, D.H.; Kim, J.Y. Conditioned Media from Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Inhibits Melanogenesis by Promoting Proteasomal Degradation of MITF. PLoS ONE 2015, 10, e0128078. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Son, Y.O.; Kook, S.H.; Choi, K.C.; Lee, J.C. Ascorbic acid increases the activity and synthesis of tyrosinase in B16F10 cells through activation of p38 mitogen-activated protein kinase. Arch. Dermatol. Res. 2011, 303, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Hayashi, N.; Kawashima, M.; Imokawa, G. A single UVB exposure increases the expression of functional KIT in human melanocytes by up-regulating MITF expression through the phosphorylation of p38/CREB. Arch. Dermatol. Res. 2010, 302, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Chu, J.H.; Wang, H.; Xu, H.; Chou, G.X.; Leung, A.K.; Fong, W.F.; Yu, Z.L. Involvement of p38 MAPK signaling pathway in the anti-melanogenic effect of San-bai-tang, a Chinese herbal formula, in B16 cells. J. Ethnopharmacol. 2010, 132, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Chang, S.J.; Wu, C.Y.; Ke, H.J.; Chang, T.M. [6]-Shogaol inhibits α-MSH-induced melanogenesis through the acceleration of ERK and PI3K/Akt-mediated MITF degradation. BioMed Res. Int. 2014, 2014, 842569. [Google Scholar] [CrossRef] [PubMed]
- Smoller, B.R.; McNutt, N.S.; Hsu, A. HMB-45 recognizes stimulated melanocytes. J. Cutan. Pathol. 1989, 16, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Busam, K.J.; Jungbluth, A.A. Melan-A, a new melanocytic differentiation marker. Adv. Anat. Pathol. 1999, 6, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.A.; Sarmidi, M.R.; Park, C.S. Mangosteen leaf extract increases melanogenesis in B16F1 melanoma cells by stimulating tyrosinase activity in vitro and by up-regulating tyrosinase gene expression. Int. J. Mol. Med. 2012, 29, 209–217. [Google Scholar] [PubMed]










| Dendritic Cell Number | Control | MSH | 30 Hz | 50 Hz | 60 Hz | 75 Hz |
|---|---|---|---|---|---|---|
| multipolar morphology | 9 ± 3 | 16 ± 3 | 11 ± 4 | 25 ± 4 | 24 ± 3 | 18 ± 4 |
| Marker | Control | MSH | 30 Hz | 50 Hz | 60 Hz | 75 Hz |
|---|---|---|---|---|---|---|
| HMB45 | + | ++ | + | +++ | +++ | ++ |
| Melan-A | + | ++ | + | +++ | +++ | ++ |
| Gene | Upstream Primer Sequence | Downstream Primer Sequence |
|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase | 5′-ACC ACA GTC CAT GCC ATC AC-3′ | 5′-TCC ACC CTG TTG CTG TA-3′ |
| Tyrosinase | 5′-CTC AAA GCA TGC ACA AT-3′ | 5′-GCC CAG ATC TTT GGA TGA AA-3′ |
| Tyrosinase-related protein 2 | 5′-TTC GGC AGA ACA TCC ATT CC-3′ | 5′-TTG GCA ATT TCA TGC TGT TTC-3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-M.; Cho, S.-E.; Kim, S.-C.; Jang, H.-J.; Seo, Y.-K. Effects of Extremely Low Frequency Electromagnetic Fields on Melanogenesis through p-ERK and p-SAPK/JNK Pathways in Human Melanocytes. Int. J. Mol. Sci. 2017, 18, 2120. https://doi.org/10.3390/ijms18102120
Kim Y-M, Cho S-E, Kim S-C, Jang H-J, Seo Y-K. Effects of Extremely Low Frequency Electromagnetic Fields on Melanogenesis through p-ERK and p-SAPK/JNK Pathways in Human Melanocytes. International Journal of Molecular Sciences. 2017; 18(10):2120. https://doi.org/10.3390/ijms18102120
Chicago/Turabian StyleKim, Yu-Mi, Sang-Eun Cho, Soo-Chan Kim, Hyun-Joon Jang, and Young-Kwon Seo. 2017. "Effects of Extremely Low Frequency Electromagnetic Fields on Melanogenesis through p-ERK and p-SAPK/JNK Pathways in Human Melanocytes" International Journal of Molecular Sciences 18, no. 10: 2120. https://doi.org/10.3390/ijms18102120
APA StyleKim, Y. -M., Cho, S. -E., Kim, S. -C., Jang, H. -J., & Seo, Y. -K. (2017). Effects of Extremely Low Frequency Electromagnetic Fields on Melanogenesis through p-ERK and p-SAPK/JNK Pathways in Human Melanocytes. International Journal of Molecular Sciences, 18(10), 2120. https://doi.org/10.3390/ijms18102120