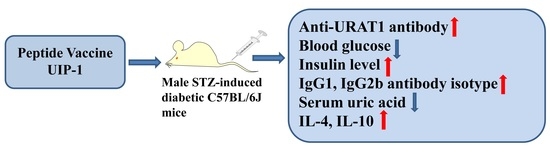
A Novel Multi-Epitope Vaccine Based on Urate Transporter 1 Alleviates Streptozotocin-Induced Diabetes by Producing Anti-URAT1 Antibody and an Immunomodulatory Effect in C57BL/6J Mice
Abstract
:1. Introduction
2. Results
2.1. Hypoglycemic Activity in Streptozotocin (STZ)-Induced Diabetes in C57BL/6J Mice
2.2. The Detection of Serum and Urine Uric Acid
2.3. Effect of U-IA-2(5)-P2-1 (UIP-1) on Total Antioxidant Capacity (T-AOC) and Superoxide Dismutase (SOD) Activity and Malondialdehyde (MDA)
2.4. Vaccine UIP-1 Protecting Islets from Damaging
2.5. The Detection of URAT1 Antibody and IgG Isotype
2.6. T Cell Proliferation and Cytokine Assay
3. Discussion
4. Materials and Methods
4.1. Vaccine Design and Synthesis
4.2. Mice
4.3. Animal Experiments
4.4. ELISA Analyses of Specific Antibodies and the Relative Affinity of Antibodies against URAT1
4.5. Urine Glucose Tests
4.6. Antibody Isotyping Analyses
4.7. Uric Acid Assay
4.8. Uric Acid Reabsorption Rates
4.9. T Cell Proliferation Assays
4.10. SDS-PAGE and Western Blot
4.11. Cytokine Assays
4.12. Measurement of SOD, T-AOC, and MDA Activity
4.13. Serum Insulin Determination
4.14. Histological Analysis and Immunohistochemical Analysis
4.15. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| ChREBP | Carbohydrate-responsive element-binding protein |
| ELISA | Enzyme-linked immunosorbent assay |
| GLU9 | Glucose transporter 9 |
| IA-2 | Insulinoma antigen-2 |
| IFN-γ | interferon-gamma |
| IL-2 | Interleukin-2 |
| IL-4 | Interleukin-4 |
| IL-10 | Interleukin-10 |
| MDA | Malondialdehyde |
| MTT | Methyltrityl |
| MSU | Monosodium urate |
| OAT1 | Organic anion transporter 1 |
| PBS | Phosphate-buffered saline |
| PBST | Phosphate-buffered saline containing 0.1% Tween-20 |
| PVDF | Polyvinylidene luoride |
| ROS | Reactive oxygen species |
| RTECS | Renal tubular epithelial cells |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SOD | Superoxide dismutase |
| STZ | Streptozotocin |
| SUA | Serum uric acid |
| SuUA | Supernatant’s uric acid |
| T-AOC | Total antioxidant capacity |
| T1DM | Type 1 Diabetes Mellitus |
| UIP-1 | U-IA-2(5)-P2-1 |
| URAT1 | Urate transporter 1 |
| UUA | Urine uric acid |
References
- Ogbera, A.O.; Azenabor, A.O. Hyperuricaemia and the metabolic syndrome in type 2 DM. Diabetol. Metab. Syndr. 2010, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Mundhe, S.; Mhasde, D. The study of prevalence of hyperuricemia and metabolic syndrome in type 2 diabetes mellitus. Int. J. Adv. Med. 2016, 3, 241–249. [Google Scholar] [CrossRef]
- Kushiyama, A.; Nakatsu, Y.; Matsunaga, Y.; Yamamotoya, T.; Mori, K.; Ueda, K.; Inoue, Y.; Sakoda, H.; Fujishiro, M.; Ono, H.; et al. Role of Uric Acid Metabolism-Related Inflammation in the Pathogenesis of Metabolic Syndrome Components Such as Atherosclerosis and Nonalcoholic Steatohepatitis. Mediat. Inflamm. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, Y.; Huang, T.; Zhang, Y.; Li, Z.; Luo, C.; Luo, Y.; Yuan, H.; Hisatome, I.; Yamamoto, T.; et al. High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochem. Biophys. Res. Commun. 2014, 447, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Martins-Santos, M.E.S.; Chaves, V.E. Uric acid as a modulator of glucose and lipid metabolism. Biochimie 2015, 116, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Viazzi, F.; Piscitelli, P.; Giorda, C.; Ceriello, A.; Genovese, S.; Russo, G.; Guida, P.; Fioretto, P.; De Cosmo, S.; Pontremoli, R.; et al. Metabolic syndrome, serum uric acid and renal risk in patients with T2D. PLoS ONE 2017, 12, e0176058. [Google Scholar] [CrossRef] [PubMed]
- Caliceti, C.; Calabria, D.; Roda, A.; Cicero, A.F.G. Fructose Intake, Serum Uric Acid, and Cardiometabolic Disorders: A Critical Review. Nutrients 2017, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Bove, M.; Cicero, A.F.G.; Veronesi, M.; Borghi, C. An evidence-based review on urate-lowering treatments: Implications for optimal treatment of chronic hyperuricemia. Vasc. Health Risk Manag. 2017, 13, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yu, C.; Li, X.; Sun, L.; Zhu, X.; Zhao, C.; Zhang, Z.; Yang, Z. Serum Uric Acid Levels and Risk of Metabolic Syndrome: A Dose-Response Meta-Analysis of Prospective Studies. J. Clin. Endocrinol. Metab. 2015, 100, 4198–4207. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.E.K.; Klein, R.; Lee, K.E. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care 2002, 25, 1790–1794. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.; Blaha, M.J.; Nasir, K.; Silverman, M.G.; Tota-Maharaj, R.; Carvalho, J.A.M.; Conceição, R.D.; Blumenthal, R.S.; Santos, R.D. Relation of Uric Acid to Serum Levels of High-Sensitivity C-Reactive Protein, Triglycerides, and High-Density Lipoprotein Cholesterol and to Hepatic Steatosis. Am. J. Cardiol. 2012, 110, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Li, C.; Cook, S.; Choi, H.K. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 2007, 115, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.W.; Sung, K.C.; Shin, H.S.; Kim, B.J.; Kim, B.S.; Kang, J.H.; Lee, M.H.; Park, J.R.; Kim, H.; Rhee, E.J.; et al. Relationship between serum uric acid concentration and insulin resistance and metabolic syndrome. Circ. J. Off. J. Jpn. Circ. Soc. 2005, 69, 928–933. [Google Scholar] [CrossRef]
- Facchini, F.; Chen, Y.D.; Hollenbeck, C.B.; Reaven, G.M. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 1991, 266, 3008–3011. [Google Scholar] [CrossRef] [PubMed]
- Rathmann, W.; Funkhouser, E.; Dyer, A.R.; Roseman, J.M. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: The CARDIA study. Coronary Artery Risk Development in Young Adults. Ann. Epidemiol. 1998, 8, 250–261. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Cicerchi, C.; Li, N.; Roncal-Jimenez, C.A.; Ishimoto, T.; Le, M.; Garcia, G.E.; Thomas, J.B.; Rivard, C.J.; et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS ONE 2012, 7, e47948. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, E.; Giampietro, O. Oxidative stress in families of type 1 diabetic patients. Diabetes Care 2000, 23, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Wakayama, T.; Mamada, H.; Shirasaka, Y.; Nakanishi, T.; Tamai, I. Identification and functional characterization of uric acid transporter Urat1 (Slc22a12) in rats. Biochim. Biophys. Acta 2011, 1808, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wei, Y.; Liu, M.; Liu, T.; Li, J.; Ji, Y.; Liang, J. Anti-hyperuricemic and nephroprotective effects of Rhizoma Dioscoreae septemlobae extracts and its main component dioscin via regulation of mOAT1, mURAT1 and mOCT2 in hypertensive mice. Arch. Pharm. Res. 2014, 37, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Endou, H. Roles of organic anion transporters (OATs) and a urate transporter (URAT1) in the pathophysiology of human disease. Clin. Exp. Nephrol. 2005, 9, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-J.; Lu, Y.; Hou, J.; Dong, Y.-K.; Du, M.-Z.; Xing, Y.; Ge, C.-Y.; Xu, M.-L.; Jin, L.; Cao, R.-Y.; et al. Vaccination of non-obese diabetic mice with a fragment of peptide P277 attenuates insulin-dependent diabetes mellitus. Int. Immunopharmacol. 2011, 11, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Piquer, S.; Valera, L.; Lampasona, V.; Jardin-Watelet, B.; Roche, S.; Granier, C.; Roquet, F.; Christie, M.R.; Giordano, T.; Malosio, M.-L.; et al. Monoclonal antibody 76F distinguishes IA-2 from IA-2β and overlaps an autoantibody epitope. J. Autoimmun. 2006, 26, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.K.; Lampasona, V.; Wyatt, R.; Brigatti, C.; Gillespie, K.M.; Bingley, P.J.; Achenbach, P. Reactivity to N-Terminally Truncated GAD65(96-585) Identifies GAD Autoantibodies That Are More Closely Associated With Diabetes Progression in Relatives of Patients With Type 1 Diabetes. Diabetes 2015, 64, 3247–3252. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.C.; Wang, B.; Tisch, R. Genetic vaccination for re-establishing T-cell tolerance in type 1 diabetes. Hum. Vaccines 2011, 7, 27–36. [Google Scholar] [CrossRef]
- Elias, D.; Meilin, A.; Ablamunits, V.; Birk, O.S.; Carmi, P.; Könen-Waisman, S.; Cohen, I.R. Hsp60 peptide therapy of NOD mouse diabetes induces a Th2 cytokine burst and downregulates autoimmunity to various β-cell antigens. Diabetes 1997, 46, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wang, Y.; Xiong, Q.; Chen, Q.; Li, J.; Zhu, A.; Cao, R.; Wu, J.; Liu, J. Long-lasting specific antibodies against P277 induced by mucosal administration of P277 repeat sequences carried by Hsp65 in the absence of adjuvants. Vaccine 2007, 25, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, J.; Song, N.; Chen, R.; Zhang, T.; Ding, X. Hyperuricemia increases the risk of acute kidney injury: A systematic review and meta-analysis. BMC Nephrol. 2017, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Harris, R.C. Renal endothelial dysfunction in diabetic nephropathy. Cardiovasc. Hematol. Disord. Drug Targets 2014, 14, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The Pathobiology of Diabetic Complications. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Matsuo, H.; Takada, T.; Nakayama, A.; Murakami, K.; Shimizu, T.; Yamanashi, Y.; Kasuga, H.; Nakashima, H.; Nakamura, T.; et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 2012, 3, 764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Du, T.; Li, M.; Lu, H.; Lin, X.; Yu, X. Combined effect of obesity and uric acid on nonalcoholic fatty liver disease and hypertriglyceridemia. Medicine 2017, 96, e6381. [Google Scholar] [CrossRef] [PubMed]
- Abeles, A.M. Hyperuricemia, gout, and cardiovascular disease: An update. Curr. Rheumatol. Rep. 2015, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Barabé, F.; Gilbert, C.; Liao, N.; Bourgoin, S.G.; Naccache, P.H. Crystal-induced neutrophil activation VI. Involvment of FcγRIIIB (CD16) and CD11b in response to inflammatory microcrystals. FASEB J. 1998, 12, 209–220. [Google Scholar] [PubMed]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Yang, F.; Yang, I.; Yin, Y.; Luo, J.J.; Wang, H.; Yang, X.-F. Uric acid, hyperuricemia and vascular diseases. Front. Biosci. Landmark Ed. 2012, 17, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Liblau, R.S.; Singer, S.M.; McDevitt, H.O. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol. Today 1995, 16, 34–38. [Google Scholar] [CrossRef]
- Todd, J.A. Etiology of Type 1 Diabetes. Immunity 2010, 32, 457–467. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar]
- Greene, M.L.; Marcus, R.; Aurbach, G.D.; Kazam, E.S.; Seegmiller, J.E. Hypouricemia due to isolated renal tubular defect. Dalmatian dog mutation in man. Am. J. Med. 1972, 53, 361–367. [Google Scholar] [CrossRef]
- Preitner, F.; Bonny, O.; Laverriere, A.; Rotman, S.; Firsov, D.; Da Costa, A.; Metref, S.; Thorens, B. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc. Natl. Acad. Sci. USA 2009, 106, 15501–15506. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Yang, H.-J.; Park, K.; Lee, J.; Kim, B.-W. Reparatory and preventive effects of oriental herb extract mixture (OHEM) on hyperuricemia and gout. Food Sci. Biotechnol. 2010, 19, 517–524. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Y.; Wang, B.; Zhang, F.; Wang, D.; Wang, Y. Allopurinol treatment improves renal function in patients with type 2 diabetes and asymptomatic hyperuricemia: 3-year randomized parallel-controlled study. Clin. Endocrinol. 2015, 83, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; MacDonald, P.A.; Hunt, B.J.; Jackson, R.L. Diabetes and gout: Efficacy and safety of febuxostat and allopurinol. Diabetes Obes. Metab. 2013, 15, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Jeong, K.H.; Kim, Y.G.; Moon, J.Y.; Lee, S.H.; Ihm, C.G.; Sung, J.Y.; Lee, T.W. Febuxostat ameliorates diabetic renal injury in a streptozotocin-induced diabetic rat model. Am. J. Nephrol. 2014, 40, 56–63. [Google Scholar] [CrossRef] [PubMed]
- López, L.C.; Varea, O.; Navarro, S.; Carrodeguas, J.A.; Sanchez de Groot, N.; Ventura, S.; Sancho, J. Benzbromarone, Quercetin, and Folic Acid Inhibit Amylin Aggregation. Int. J. Mol. Sci. 2016, 17, 964. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, T.; Zhao, J.; Sun, P.; Yan, G.; Ding, H.; Zhu, W.; Chen, K. Benzbromarone, an old uricosuric drug, inhibits human fatty acid binding protein 4 in vitro and lowers the blood glucose level in db/db mice. Acta Pharmacol. Sin. 2013, 34, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ueda, Y.; Miyazawa, H.; Kaku, Y.; Hirai, K.; Hoshino, T.; Nabata, A.; Mori, H.; Yoshida, I.; Ookawara, S.; et al. Acute severe liver dysfunction induced by febuxostat in a patient undergoing hemodialysis. CEN Case Rep. 2014, 3, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Bardin, T. Current management of gout in patients unresponsive or allergic to allopurinol. Jt. Bone Spine 2004, 71, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Nivorozhkin, A.; Szabó, C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol. Rev. 2006, 58, 87–114. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.-S.; Kim, M.J.; Shin, H.-S.; Jang, Y.-H.; Choi, H.S.; Jo, I.; Johnson, R.J.; Kang, D.-H. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2013, 304, F471–F480. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.A.; Sliem, H.A.; Ellethy, A.T.; Salama, M.E.-S. Role of immune system modulation in prevention of type 1 diabetes mellitus. Indian J. Endocrinol. Metab. 2012, 16, 904–909. [Google Scholar] [PubMed]
- Ichida, K.; Hosoyamada, M.; Hisatome, I.; Enomoto, A.; Hikita, M.; Endou, H.; Hosoya, T. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J. Am. Soc. Nephrol. 2004, 15, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhu, A.; Wang, Y.; Liu, J. Enhanced immunogenicity of peptide P277 by heat shock protein HSP65 vector carrying tandem repeats of P277 to prevent type 1 diabetes in NOD mice. Exp. Clin. Endocrinol. Diabetes 2008, 116, 541–548. [Google Scholar]
- Jin, L.; Zhu, A.; Wang, Y.; Lu, Y.; Liu, J. HSP65 serves as an immunogenic carrier for a diabetogenic peptide P277 inducing anti-inflammatory immune response in NOD mice by nasal administration. Vaccine 2010, 28, 3312–3317. [Google Scholar]
- Lu, S.; Li, G.; Liu, K.; Yang, X.; Cao, R.; Zong, L.; Long, J.; Jin, L.; Wu, J. Fusion protein His-Hsp65-6IA2P2 prevents type 1 diabetes through nasal immunization in NOD Mice. Int. Immunopharmacol. 2016, 35, 235–242. [Google Scholar] [CrossRef] [PubMed]






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Cao, H.; Li, Z.; Fang, J.; Wei, X.; Cheng, P.; Jiao, R.; Liu, X.; Li, Y.; Xing, Y.; et al. A Novel Multi-Epitope Vaccine Based on Urate Transporter 1 Alleviates Streptozotocin-Induced Diabetes by Producing Anti-URAT1 Antibody and an Immunomodulatory Effect in C57BL/6J Mice. Int. J. Mol. Sci. 2017, 18, 2137. https://doi.org/10.3390/ijms18102137
Ma Y, Cao H, Li Z, Fang J, Wei X, Cheng P, Jiao R, Liu X, Li Y, Xing Y, et al. A Novel Multi-Epitope Vaccine Based on Urate Transporter 1 Alleviates Streptozotocin-Induced Diabetes by Producing Anti-URAT1 Antibody and an Immunomodulatory Effect in C57BL/6J Mice. International Journal of Molecular Sciences. 2017; 18(10):2137. https://doi.org/10.3390/ijms18102137
Chicago/Turabian StyleMa, Yanjie, Huimin Cao, Zhixin Li, Jinzhi Fang, Xiaomin Wei, Peng Cheng, Rui Jiao, Xiaoran Liu, Ya Li, Yun Xing, and et al. 2017. "A Novel Multi-Epitope Vaccine Based on Urate Transporter 1 Alleviates Streptozotocin-Induced Diabetes by Producing Anti-URAT1 Antibody and an Immunomodulatory Effect in C57BL/6J Mice" International Journal of Molecular Sciences 18, no. 10: 2137. https://doi.org/10.3390/ijms18102137
APA StyleMa, Y., Cao, H., Li, Z., Fang, J., Wei, X., Cheng, P., Jiao, R., Liu, X., Li, Y., Xing, Y., Tang, J., Jin, L., & Li, T. (2017). A Novel Multi-Epitope Vaccine Based on Urate Transporter 1 Alleviates Streptozotocin-Induced Diabetes by Producing Anti-URAT1 Antibody and an Immunomodulatory Effect in C57BL/6J Mice. International Journal of Molecular Sciences, 18(10), 2137. https://doi.org/10.3390/ijms18102137