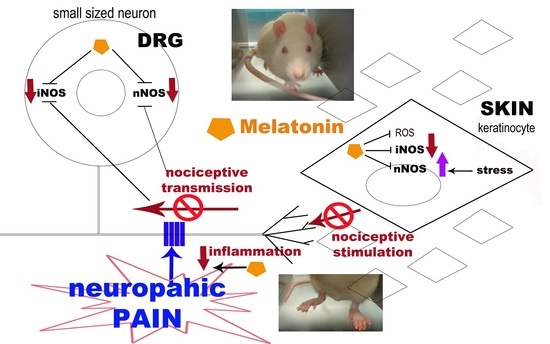
Single Administration of Melatonin Modulates the Nitroxidergic System at the Peripheral Level and Reduces Thermal Nociceptive Hypersensitivity in Neuropathic Rats
Abstract
:1. Introduction
2. Results
2.1. Behavioural Test: Thermal Hyperalgesia
2.2. nNOS and iNOS Immunohistochemistry
2.2.1. Dorsal Root Ganglia (DRG) Small Neurons
nNOS Staining
iNOS Staining
2.2.2. Hind-Paw Skin Epidermis
nNOS Staining
iNOS Staining
3. Materials and Methods
3.1. Animal Treatment
3.2. Behavioural Test: Thermal Hyperalgesia
3.3. Immunohistochemical Evaluations
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rodella, L.F.; Borsani, E.; Rezzani, R.; Ricci, F.; Buffoli, B.; Bianchi, R. AM404, an inhibitor of anandamide reuptake decreases Fos-immunoreactivity in the spinal cord of neuropathic rats after non-noxious stimulation. Eur. J. Pharmacol. 2005, 508, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Martucci, C.; Trovato, A.E.; Costa, B.; Borsani, E.; Franchi, S.; Magnaghi, V.; Panerai, A.E.; Rodella, L.F.; Valsecchi, A.E.; Sacerdote, P.; et al. The purinergic antagonist PPADS reduces pain related behaviours and interleukin-1 beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain 2008, 137, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Borsani, E.; Albertini, R.; Colleoni, M.; Sacerdote, P.; Trovato, A.E.; Lonati, C.; Labanca, M.; Panerai, A.E.; Rezzani, R.; Rodella, L.F. PPADS, a purinergic antagonist reduces Fos expression at spinal cord level in a mouse model of mononeuropathy. Brain Res. 2008, 1199, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Buffoli, B.; Borsani, E.; Rezzani, R.; Rodella, L.F. Chronic constriction injury induces aquaporin-2 expression in the dorsal root ganglia of rats. J. Anat. 2009, 215, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Maftei, D.; Marconi, V.; Florenzano, F.; Giancotti, L.A.; Castelli, M.; Moretti, S.; Borsani, E.; Rodella, L.F.; Balboni, G.; Luongo, L.; et al. Controlling the activation of the Bv8/prokineticin system reduces neuroinflammation and abolishes thermal and tactile hyperalgesia in neuropathic animals. Br. J. Pharmacol. 2014, 171, 4850–4865. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, R.; Maftei, D.; Marconi, V.; Florenzano, F.; Franchi, S.; Borsani, E.; Rodella, L.F.; Balboni, G.; Salvadori, S.; Sacerdote, P.; et al. Prokineticin 2 upregulation in the peripheral nervous system has a major role in triggering and maintaining neuropathic pain in the chronic constriction injury model. Biomed. Res. Int. 2015, 2015, 301292. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Barr, T.; Gee, L.; Vickers, J.; Wymer, J.; Borsani, E.; Rodella, L.; Getsios, S.; Burdo, T.; Eisenberg, E.; et al. Keratinocyte expression of calcitonin gene-related peptide β: Implications for neuropathic and inflammatory pain mechanisms. Pain 2011, 152, 2036–2051. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Sakamoto, T.; Tiwari, V.; Kim, Y.S.; Yang, F.; Dong, X.; Güler, A.D.; Guan, Y.; Caterina, M.J. Selective keratinocyte stimulation is sufficient to evoke nociception in mice. Pain 2015, 156, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Roosterman, D.; Goerge, T.; Schneider, S.W.; Bunnett, N.W.; Steinhoff, M. Neuronal control of skin function: The skin as a neuroimmunoendocrine organ. Physiol. Rev. 2006, 86, 1309–1379. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Calvo, J.R.; Abreu, P.; Lardone, P.J.; García-Mauriño, S.; Reiter, R.J.; Guerrero, J.M. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: Possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004, 18, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. 2008, 19, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J.; Conti, A.; Pierpaoli, W. Role of the pineal gland in immunity. Circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J. Neuroimmunol. 1986, 13, 19–30. [Google Scholar] [CrossRef]
- Conti, A.; Conconi, S.; Hertens, E.; Skwarlo-Sonta, K.; Markowska, M.; Maestroni, J.M. Evidence for melatonin synthesis in mouse and human bone marrow cells. J. Pineal Res. 2000, 28, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P.; Gögenur, I.; Fenger, A.Q.; Petersen, M.C.; Rosenberg, J.; Werner, M.U. Analgesic and antihyperalgesic effects of melatonin in a human inflammatory pain model: A randomized, double-blind, placebo-controlled, three-arm crossover study. Pain 2015, 156, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.B.; Hsieh, M.C.; Lai, C.Y.; Cheng, J.K.; Wang, H.H.; Chau, Y.P.; Chen, G.D.; Peng, H.Y. Melatonin relieves neuropathic allodynia through spinal MT2-enhanced PP2Ac and downstream HDAC4 shuttling-dependent epigenetic modification of hmgb1 transcription. J. Pineal Res. 2016, 60, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Aversa, S.; Arrigo, T.; Reiter, R.J.; Gitto, E. Analgesic, anxiolytic and anaesthetic effects of melatonin: New potential uses in pediatrics. Int. J. Mol. Sci. 2015, 16, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Baker, M.T.; Spadoni, G.; Gregerson, M. The hypnotic and analgesic effects of 2-bromomelatonin. Anesth. Analg. 2003, 97, 763–768. [Google Scholar] [CrossRef] [PubMed]
- El-Shenawy, S.M.; Abdel-Salam, O.M.; Baiuomy, A.R.; El-Batran, S.; Arbid, M.S. Studies on the anti-inflammatory and anti-nociceptive effects of melatonin in the rat. Pharmacol. Res. 2002, 46, 235–243. [Google Scholar] [CrossRef]
- Zahn, P.K.; Lansmann, T.; Berger, E.; Speckmann, E.J.; Musshoff, U. Gene expression and functional characterization of melatonin receptors in the spinal cord of the rat: Implications for pain modulation. J. Pineal Res. 2003, 35, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, V.; Agrewala, J.N.; Kulkarni, S.K. Melatonin reversal of lipopolysacharides-induced thermal and behavioral hyperalgesia in mice. Eur. J. Pharmacol. 2000, 395, 15–21. [Google Scholar] [CrossRef]
- Kumar, A.; Meena, S.; Kalonia, H.; Gupta, A.; Kumar, P. Effect of nitric oxide in protective effect of melatonin against chronic constriction sciatic nerve injury induced neuropathic pain in rats. Indian J. Exp. Biol. 2011, 49, 664–671. [Google Scholar] [PubMed]
- Seyit, D.A.; Degirmenci, E.; Oguzhanoglu, A. Evaluation of Electrophysiological Effects of Melatonin and Alpha Lipoic Acid in Rats with Streptozotocine Induced Diabetic Neuropathy. Exp. Clin. Endocrinol. Diabetes 2016, 124, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Kahya, M.C.; Nazıroğlu, M.; Övey, İ.S. Modulation of Diabetes-Induced Oxidative Stress, Apoptosis, and Ca2+ Entry Through TRPM2 and TRPV1 Channels in Dorsal Root Ganglion and Hippocampus of Diabetic Rats by Melatonin and Selenium. Mol. Neurobiol. 2017, 54, 2345–2360. [Google Scholar] [CrossRef] [PubMed]
- Areti, A.; Komirishetty, P.; Akuthota, M.; Malik, R.A.; Kumar, A. Melatonin prevents mitochondrial dysfunction and promotes neuroprotection by inducing autophagy during oxaliplatin-evoked peripheral neuropathy. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Weaver, D.R.; Rivkees, S.A.; Reppert, S.M. Localization and characterization of melatonin receptors in rodent brain by in vitro autoradiography. J. Neurosci. 1989, 9, 2581–2590. [Google Scholar] [PubMed]
- Williams, L.M.; Hannah, L.T.; Hastings, M.H.; Maywood, E.S. Melatonin receptors in the rat brain and pituitary. J. Pineal Res. 1995, 19, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Liao, M.; Brown, G.M.; Pang, S.F. Localization and characterization of melatonin receptors in the rabbit spinal cord. Neurosci. Lett. 1996, 204, 77–80. [Google Scholar] [CrossRef]
- Fischer, T.W.; Slominski, A.; Zmijewski, M.A.; Reiter, R.J.; Paus, R. Melatonin as a major skin protectant: From free radical scavenging to DNA damage repair. Exp. Dermatol. 2008, 17, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.X.; Zhu, B.; Xu, S.F.; Cao, X.D.; Wu, G.C. The analgesic effects of peripheral and central administration of melatonin in rats. Eur. J. Pharmacol. 2000, 403, 49–53. [Google Scholar] [CrossRef]
- Laurido, C.; Pelissie, T.; Soto-Moyano, R.; Valladares, L.; Flores, F.; Hernández, A. Effect of melatonin on rat spinal cord nociceptive transmission. Neuroreport 2002, 13, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Ulugol, A.; Dokmeci, D.; Guray, G.; Sapolyo, N.; Ozyigit, F.; Tamer, M. Antihyperalgesic, but not antiallodynic, effect of melatonin in nerve-injured neuropathic mice: Possible involvements of the L-arginine-NO pathway and opioid system. Life Sci. 2006, 78, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Cals-Grierson, M.M.; Ormerod, A.D. Nitric oxide function in the skin. Nitric Oxide 2004, 10, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Galeano, R.M.; Germanà, A.; Vázquez, M.T.; Hidaka, H.; Germanà, G.; Vega, J.A. Immunohistochemical localization of neurocalcin in human sensory neurons and mechanoreceptors. Neurosci. Lett. 2000, 279, 89–92. [Google Scholar] [CrossRef]
- Ng Wing Tin, S.; Ciampi de Andrade, D.; Goujon, C.; Planté-Bordeneuve, V.; Créange, A.; Lefaucheur, J.P. Sensory correlates of pain in peripheral neuropathies. Clin. Neurophysiol. 2014, 125, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Kojundzic, S.L.; Dujmovic, I.; Grkovic, I.; Sapunar, D. Regional differences in epidermal thickness and behavioral response following partial denervation of the rat paw. Int. J. Neurosci. 2008, 118, 1748–1762. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.P.; Pini, A.; Evan, G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 1987, 328, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Catheline, G.; Le Guen, S.; Honoré, P.; Besson, J.M. Are there long-term changes in the basal or evoked Fos expression in the dorsal horn of the spinal cord of the mononeuropathic rat? Pain 1999, 80, 347–357. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Chang, H.M.; Ling, E.A.; Chen, C.F.; Lue, H.; Wen, C.Y.; Shieh, J.Y. Melatonin attenuates the neuronal NADPH-d/NOS expression in the nodose ganglion of acute hypoxic rats. J. Pineal Res. 2002, 32, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Coronel, M.F.; Brumovsky, P.R.; Hökfelt, T.; Villar, M.J. Differential galanin upregulation in dorsal root ganglia and spinal cord after graded single ligature nerve constriction of the rat sciatic nerve. J. Chem. Neuroanat. 2008, 35, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Shen, H.; Taglialatela, G.; Chung, J.M.; Chung, K. Expression of nerve growth factor in the dorsal root ganglion after peripheral nerve injury. Brain Res. 1998, 796, 99–106. [Google Scholar] [CrossRef]
- Borsani, E.; Giovannozzi, S.; Boninsegna, R.; Rezzani, R.; Labanca, M.; Tschabitscher, M.; Rodella, L.F. Nitroxidergic system in human trigeminal ganglia neurons: A quantitative evaluation. Acta Histochem. 2010, 112, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Tututi, M.; Granados-Soto, V. Oral and spinal melatonin reduces tactile allodynia in rats via activation of MT2 and opioid receptors. Pain 2007, 132, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zurowski, D.; Nowak, L.; Machowska, A.; Wordliczek, J.; Thor, P.J. Exogenous melatonin abolishes mechanical allodynia but not thermal hyperalgesia in neuropathic pain. The role of the opioid system and benzodiazepine-gabaergic mechanism. J. Physiol. Pharmacol. 2012, 63, 641–647. [Google Scholar] [PubMed]
- Nahleh, Z.; Pruemer, J.; Lafollette, J.; Sweany, S. Melatonin, a promising role intaxane-related neuropathy. Clin. Med. Insights Oncol. 2010, 4, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Paolorossi, F.; Ardizzoia, A.; Barni, S.; Chilelli, M.; Mancuso, M.; Tancini, G.; Conti, A.; Maestroni, G.J. A randomized study of chemotherapy with cisplatin plus etoposide versus chemoendocrine therapy with cisplatin, etoposide and the pineal hormone melatonin as a first-line treatment of advanced non-small cell lung cancer patients in a poor clinical state. J. Pineal Res. 1997, 23, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, F.; Seet, E.; Venkatraghavan, L.; Abrishami, A.; Chung, F. Efficacy and safety of melatonin as an anxiolytic and analgesic in the perioperative period: A qualitative systematic review of randomized trials. Anesthesiology 2010, 113, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Zakaria, R.; Jeet Singh, H.; Acuna-Castroviejo, D. Melatonin and its agonists in pain modulation and its clinical application. Arch. Ital. Biol. 2012, 150, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Bilici, D.; Akpinar, E.; Kiziltunç, A. Protective effect of melatonin in carrageenan-induced acute local inflammation. Pharmacol. Res. 2002, 46, 133–139. [Google Scholar] [CrossRef]
- Costantino, G.; Cuzzocrea, S.; Mazzon, E.; Caputi, A.P. Protective effects of melatonin in zymosan-activated plasma-induced paw inflammation. Eur. J. Pharmacol. 1998, 363, 57–63. [Google Scholar] [CrossRef]
- Hernández-Pacheco, A.; Araiza-Saldaña, C.I.; Granados-Soto, V.; Mixcoatl-Zecuatl, T. Possible participation of the nitric oxide-cyclic GMP-protein kinase G-K+ channels pathway in the peripheral antinociception of melatonin. Eur. J. Pharmacol. 2008, 596, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, M.; Kaster, M.P.; Pertile, R.; Calixto, J.B.; Rodrigues, A.L.; Santos, A.R. Mechanisms involved in the antinociception caused by melatonin in mice. J. Pineal Res. 2006, 41, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Raja, S.N.; Moore, L.C.; Tobin, J.R. Neuropathic pain in rats is associated with altered nitric oxide synthase activity in neural tissue. J. Neurol. Sci. 1996, 138, 14–20. [Google Scholar] [CrossRef]
- Cizkova, D.; Marsala, J.; Lukacova, N.; Marsala, M.; Jergova, S.; Orendacova, J.; Yaksh, T.L. Localization of N-type Ca2+ channels in the rat spinal cord following chronic constrictive nerve injury. Exp. Brain Res. 2002, 147, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Borsani, E.; Albertini, R.; Labanca, M.; Lonati, C.; Rezzani, R.; Rodella, L.F. Peripheral purinergic receptor modulation influences the trigeminal ganglia nitroxidergic system in an experimental murine model of inflammatory orofacial pain. J. Neurosci. Res. 2010, 88, 2715–2726. [Google Scholar] [CrossRef] [PubMed]
- Weller, R. Nitric oxide: A key mediator in cutaneous physiology. Clin. Exp. Dermatol. 2003, 28, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, G.G.; Parada, C.A.; Ferreira, S.H. Opposite nociceptive effects of the arginine/NO/cGMP pathway stimulation in dermal and subcutaneous tissues. Br. J. Pharmacol. 2003, 138, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Eþrefoðlu, M.; Seyhan, M.; Gül, M.; Parlakpinar, H.; Batçioðlu, K.; Uyumlu, B. Potent therapeutic effect of melatonin on aging skin in pinealectomized rats. J. Pineal Res. 2005, 39, 231–237. [Google Scholar] [CrossRef]
- Arese, M.; Magnifico, M.C.; Mastronicola, D.; Altieri, F.; Grillo, C.; Blanck, T.J.; Sarti, P. Nanomolar melatonin enhances nNOS expression and controls HaCaT-cells bioenergetics. IUBMB Life 2012, 64, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Sarti, P.; Magnifico, M.C.; Altieri, F.; Mastronicola, D.; Arese, M. New evidence for cross talk between melatonin and mitochondria mediated by a circadian-compatible interaction with nitric oxide. Int. J. Mol. Sci. 2013, 14, 11259–11276. [Google Scholar] [CrossRef] [PubMed]
- Turgut, M.; Erdogan, S.; Ergin, K.; Serte, M. Melatonin ameliorates blood-brain barrier permeability, glutathione, and nitric oxide levels in the choroid plexus of the infantile rats with kaolin-induced hydrocephalus. Brain Res. 2007, 1175, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Osuna, C.; Gitto, E. Actions of melatonin in the reduction of oxidative stress. A review. J. Biomed. Sci. 2000, 7, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Paredes, S.D.; Manchester, L.C.; Tan, D.X. Reducing oxidative/nitrosative stress: A newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; León, J.; López, L.C.; Acuña-Castroviejo, D. Mechanisms of N-methyl-D-aspartate receptor inhibition by melatonin in the rat striatum. J. Neuroendocrinol. 2004, 16, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Ayar, A.; Martin, D.J.; Ozcan, M.; Kelestimur, H. Melatonin inhibits high voltage activated calcium currents in cultured rat dorsal root ganglion neurones. Neurosci. Lett. 2001, 313, 73–77. [Google Scholar] [CrossRef]
- Pozo, D.; Reiter, R.J.; Calvo, J.R.; Guerrero, J.M. Physiological concentrations of melatonin inhibit nitric oxide synthase in rat cerebellum. Life Sci. 1994, 55, PL455–PL460. [Google Scholar] [CrossRef]
- Bettahi, I.; Pozo, D.; Osuna, C.; Reiter, R.J.; Acuña-Castroviejo, D.; Guerrero, J.M. Melatonin reduces nitric oxide synthase activity in rat hypothalamus. J. Pineal Res. 1996, 20, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Sadeghi, K.; Reiter, R.J.; Meltz, M.L. The neurohormone melatonin inhibits cytokine, mitogen and ionizing radiation induced NF-kappa B. Biochem. Mol. Biol. Int. 1995, 37, 1063–1070. [Google Scholar] [PubMed]
- Cuzzocrea, S.; Zingarelli, B.; Gilad, E.; Hake, P.; Salzman, A.L.; Szabó, C. Protective effect of melatonin in carrageenan-induced models of local inflammation: Relationship to its inhibitory effect on nitric oxide production and its peroxynitrite scavenging activity. J. Pineal Res. 1997, 23, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Salzman, A.; Denenberg, A.G.; Ueta, I.; O’Connor, M.; Linn, S.C.; Szabó, C. Induction and activity of nitric oxide synthase in cultured human intestinal epithelial monolayers. Am. J. Physiol. 1996, 270, G565–G573. [Google Scholar] [PubMed]
- Alonso, M.; Collado, P.S.; González-Gallego, J. Melatonin inhibits the expression of the inducible isoform of nitric oxide synthase and nuclear factor kappa B activation in rat skeletal muscle. J. Pineal Res. 2006, 41, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Westman, J.; Alm, P.; Sjöquist, P.O.; Cervós-Navarro, J.; Nyberg, F. Involvement of nitric oxide in the pathophysiology of acute heat stress in the rat. Influence of a new antioxidant compound H-290/51. Ann. N. Y. Acad. Sci. 1997, 813, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.H.; Hanley, D.F.; Trapp, B.D.; Saito, S.; Raja, S.; Dawson, T.M.; Yamaguchi, H. Induction of spinal cord neuronal nitric oxide synthase (NOS) after formalin injection in the rat hind paw. Neurosci. Lett. 1996, 210, 201–204. [Google Scholar] [CrossRef]
- Herdegen, T.; Brecht, S.; Mayer, B.; Leah, J.; Kummer, W.; Bravo, R.; Zimmermann, M. Long-lasting expression of JUN and KROX transcription factors and nitric oxide synthase in intrinsic neurons of the rat brain following axotomy. J. Neurosci. 1993, 13, 4130–4145. [Google Scholar] [PubMed]
- Tascedda, F.; Molteni, R.; Racagni, G.; Riva, M.A. Acute and chronic changes in K(+)-induced depolarization alter NMDA and nNOS gene expression in cultured cerebellar granule cells. Brain Res. Mol. Brain Res. 1996, 40, 171–174. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kromminga, A.; Dunlop, T.W.; Tychsen, B.; Conrad, F.; Suzuki, N.; Memezawa, A.; Bettermann, A.; Aiba, S.; Carlberg, C.; Paus, R. A role of melatonin in neuroectodermal-mesodermal interactions: The hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 2005, 19, 1710–1712. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G.; Zamponi, G.W. Regulating excitability of peripheral afferents: Emerging ion channel targets. Nat. Neurosci. 2014, 17, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Wiesenfeld-Hallin, Z. Sex differences in pain perception. Gend. Med. 2005, 2, 137–145. [Google Scholar] [CrossRef]
- Frye, C.A.; Cuevas, C.A.; Kanarek, R.B. Diet and estrous cycle influence pain sensitivity in rats. Pharmacol. Biochem. Behav. 1993, 45, 255–260. [Google Scholar] [CrossRef]








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsani, E.; Buffoli, B.; Bonazza, V.; Reiter, R.J.; Rezzani, R.; Rodella, L.F. Single Administration of Melatonin Modulates the Nitroxidergic System at the Peripheral Level and Reduces Thermal Nociceptive Hypersensitivity in Neuropathic Rats. Int. J. Mol. Sci. 2017, 18, 2143. https://doi.org/10.3390/ijms18102143
Borsani E, Buffoli B, Bonazza V, Reiter RJ, Rezzani R, Rodella LF. Single Administration of Melatonin Modulates the Nitroxidergic System at the Peripheral Level and Reduces Thermal Nociceptive Hypersensitivity in Neuropathic Rats. International Journal of Molecular Sciences. 2017; 18(10):2143. https://doi.org/10.3390/ijms18102143
Chicago/Turabian StyleBorsani, Elisa, Barbara Buffoli, Veronica Bonazza, Russel J. Reiter, Rita Rezzani, and Luigi F. Rodella. 2017. "Single Administration of Melatonin Modulates the Nitroxidergic System at the Peripheral Level and Reduces Thermal Nociceptive Hypersensitivity in Neuropathic Rats" International Journal of Molecular Sciences 18, no. 10: 2143. https://doi.org/10.3390/ijms18102143
APA StyleBorsani, E., Buffoli, B., Bonazza, V., Reiter, R. J., Rezzani, R., & Rodella, L. F. (2017). Single Administration of Melatonin Modulates the Nitroxidergic System at the Peripheral Level and Reduces Thermal Nociceptive Hypersensitivity in Neuropathic Rats. International Journal of Molecular Sciences, 18(10), 2143. https://doi.org/10.3390/ijms18102143