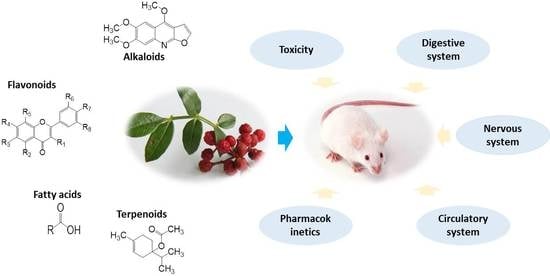
Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology
Abstract
:1. Introduction
2. Traditional Usages
3. Botany
4. Phytochemistry
4.1. Alkaloids (1–35)
4.1.1. Alkylamides
4.1.2. Other Alkaloids
4.2. Terpenoids (36–103)
4.3. Flavonoids (104–129)
4.4. Fatty Acids (130–139)
4.5. Others (140–149)
5. Pharmacology
5.1. Effect on the Digestive System
5.2. Effect on the Nervous System
5.3. Effect on the Circulatory System
5.4. Anti-Inflammatory and Analgesic Effects
5.5. Antioxidant Effect
5.6. Anti-Tumor Effect
5.7. Anti-Bacterial and Anti-Fungal Effects
5.8. Insecticidal Effects
5.9. Other Pharmacological Effects
5.10. Summary of Pharmacologic Effects
6. Pharmacokinetics
7. Toxicology
8. Future Perspectives and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AEEZBL | acetone fraction of the ethanol extraction of Z. bungeanum leaves |
| AZB | alkaloids of Z. bungeanum |
| AZBL | acetone fraction of Z. bungeanum leaves |
| CZBL | chloroform fraction of Z. bungeanum leaves |
| EAEEZBL | ethyl acetate fraction of the ethanol extraction of Z. bungeanum leaves |
| EAZBL | ethyl acetate fraction of Z. bungeanum leaves |
| EEZB | ethanol extracts of Z. bungeanum |
| EEZBL | ethanol extracts of Z. bungeanum leaves |
| EFZBL | ethanol fraction of Z. bungeanum leaves |
| EOZB | essential oils of Z. bungeanum |
| EZBL | extract of Z. bungeanum leaves |
| EZBP | extract of Z. bungeanum pericarps |
| FAEZBL | flavonoids of acetone extraction of Z. bungeanum leaves |
| FAZB | fatty acids of Z. bungeanum |
| FEEZBL | flavonoids of ethanol extraction of Z. bungeanum leaves |
| FWEZBL | flavonoids of water extraction of Z. bungeanum leaves |
| gx-50 | (E)-N-[2-(3,4-dimethoxyphenyl)ethyl]-3-phenylacrylamide |
| HAS | hydroxyl-α-sanshool |
| MEEZBL | methanol fraction of the ethanol extraction of Z. bungeanum leaves |
| MEZB | methanol extracts of Z. bungeanum |
| MZBL | methanol fraction of Z. bungeanum leaves |
| PEEZB | petroleum ether extracts of Z. bungeanum |
| PEZB | polyphenol extracts of Z. bungeanum |
| PEZBL | petroleum ether fraction of Z. bungeanum leaves |
| PZB | polysaccharide of Z. bungeanum |
| PZBSK | polypeptide of Z. bungeanum seeds kernel |
| SOZB | seeds oil of Z. bungeanum |
| SZB | seeds of Z. bungeanum |
| WEZB | water extracts of Z. bungeanum |
| ZP-amide A | (2E,7E,9E)-N-(2-hydroxy-2-methylpropyl)-11-ethoxy-6-hydroxy-dodeca-2,7,9-trienamide |
| ZP-amide B | (10RS,11SR)-(2E,6Z,8E)-10,11-dihydroxy-N-(2-hydroxy-2-methylpropyl)-2,6,8-dodecatrienamide |
| ZP-amide C | (10RS,11RS)-(2E,6Z,8E)-10,11-dihydroxy-N-(2-hydroxy-2-methylpropyl)-2,6,8-dodecatrienamide |
| ZP-amide D | (2E,4E,9E,11E)-N-(2-hydroxy-2-methypropyl)-8-hydroxy-13-oxo-2,4,9,11-tetradecatetraenamide |
| ZP-amide E | tetrahydrobungeanool |
| ZP-amide F | (6RS)-(2E,7E,9E)-6-hydroxy-N-(2-hydroxy-2-methylpropyl)-11-oxo-2,7,9-dodecatrienamide |
| ZP-amide G | (2E,7E,9E)-N-(2-hydroxy-2-methylpropyl)-6,11-dioxo-2,7,9-dodecatrienamide |
References
- Wang, S.; Xie, J.C.; Yang, W.; Sun, B.G. Preparative separation and purification of alkylamides from Zanthoxylum bungeanum Maxim. by high-speed counter-current chromatography. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2640–2652. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, Y.; Xie, C.X.; Li, X.W.; Yu, Y.D.; Ye, M.; Chen, S.L. The chemical and genetic characteristics of Szechuan Pepper (Zanthoxylum bungeanum and Z. armatum) cultivars and their suitable habitat. Front. Plant Sci. 2016, 7, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Zheng, X.Y.; Kan, J.Q.; Guo, J. Evaluation of specific quality of Zanthoxylum bungeanum Maxim. and Zanthoxylum schinifolium Sieb. et Zucc. Food Sci. 2009, 30, 45–48. (In Chinese) [Google Scholar]
- Yang, F.X.; Yang, F.X.; Su, Y.Q.; Li, X.H.; Zhang, Q.; Sun, R.C. Studies on the preparation of biodiesel from Zanthoxylum bungeanum Maxim. seed oil. J. Agric. Food Chem. 2008, 56, 7891–7896. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia; Science and Technology Press of Shanghai: Shanghai, China, 1977; p. 275. (In Chinese) [Google Scholar]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia; Science and Technology Press of Shanghai: Shanghai, China, 2010; p. 149. (In Chinese) [Google Scholar]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia; Science and Technology Press of Shanghai: Shanghai, China, 2015; pp. 159–160. (In Chinese) [Google Scholar]
- Xiong, Q.B.; Shi, D.; Yamamoto, H.; Mizuo, M. Alkylamides from pericarps of Zanthoxylum bungeanum. Phytochemistry 1997, 46, 1123–1126. [Google Scholar] [CrossRef]
- Yang, X. Aroma constituents and alkylamides of red and green huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J. Agric. Food Chem. 2008, 56, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.C.; Li, R.; Tan, J.; Jiang, Z.T. Polyphenolics composition of the leaves of Zanthoxylum bungeanum Maxim. grown in Hebei, China, and their radical scavenging activities. J. Agric. Food Chem. 2013, 61, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; You, J.M.; Li, G.L.; Sun, Z.W.; Suo, Y.R. Compositional and antioxidant activity analysis of Zanthoxylum bungeanum seed oil obtained by supercritical CO2 fluid extraction. J. Am. Oil Chem. Soc. 2011, 88, 23–32. [Google Scholar] [CrossRef]
- Wei, G.C.; Zheng, A.W.; Jing, R.J.; Xie, H.B.; Chang, X.Y. Determination of five inorganic elements in Zanthoxylum bungeanum Maxim. Guangdong Trace Elem. Sci. 2008, 15, 38–41. (In Chinese) [Google Scholar] [CrossRef]
- Huang, D.M.; Zhao, G.H.; Chen, Z.D.; Kan, J.Q. The food culture of Zanthoxylum bungeanum in China. China Condiment 2016, 1, 75–81. (In Chinese) [Google Scholar] [CrossRef]
- Wang, X.T. Processed Methods of Traditional Chinese Medicine; Science and Technology Press of Jiangxi: Nanchang, China, 1989; pp. 227–228. (In Chinese) [Google Scholar]
- Zhonghua bencao Commission. Chinese Materia Medica; Science and Technology Press of Shanghai: Shanghai, China, 1999; p. 976. (In Chinese) [Google Scholar]
- Tong, X.R.; Wang, P.M. A preliminary discussion on the Wentongsanjie effect of Zanthoxylum bungeanum. LiShiZhen Med. Mater. Med. Res. 1999, 12, 897–898. (In Chinese) [Google Scholar] [CrossRef]
- Nanjing University of Traditional Chinese Medicine. Chinese Medicine Dictionary; Science and Technology Press of Shanghai: Shanghai, China, 2006; p. 1469. (In Chinese) [Google Scholar]
- Bautista, D.M.; Sigal, Y.M.; Milstein, A.D.; Garrison, J.L.; Zorn, J.A.; Tsuruda, P.R.; Nicoll, R.A.; Julius, D. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat. Neurosci. 2008, 11, 772–779. [Google Scholar] [CrossRef] [PubMed]
- State Drug Administration. Zhongyao Chengfang Zhiji; Science and Technology Press of Shanghai: Shanghai, China, 2002. (In Chinese)
- Chinese Pharmacopoeia Commission. Buban Biaozhun; Science and Technology Press of Shanghai: Shanghai, China, 1998. (In Chinese) [Google Scholar]
- Song, M.X.; Guo, W.J. Guojia Zhongchengyao; People’s Medical Publishing House: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Zhang, J.H.; Jiang, L.F. Acid-catalyzed esterification of Zanthoxylum bungeanum seed oil with high free fatty acids for biodiesel production. Bioresour. Technol. 2008, 99, 8995–8998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Wang, D.M.; Yang, L.N.; Zhou, D.; Zhang, J.F. Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity. PLoS ONE 2014, 9, e105725. [Google Scholar] [CrossRef] [PubMed]
- Chinese Flora Commission. Flora of China; Science Press: Beijing, China, 1997; p. 44. (In Chinese) [Google Scholar]
- Li, J.K.; Hui, T.; Wang, F.L.; Li, S.; Cui, B.W.; Cui, Y.Q.; Peng, Z.Q. Chinese red pepper (Zanthoxylum bungeanum, Maxim.) leaf extract as natural antioxidants in salted silver carp (Hypophthalmichthys molitrix) in dorsal and ventral muscles during processing. Food Control 2015, 56, 9–17. [Google Scholar] [CrossRef]
- Tian, J.M.; Wang, Y.; Xu, Y.Z.; Yu, Z.C.; Wei, A.Z.; Zhang, W.M.; Gao, J.M. Characterization of isobutylhydroxyamides with NGF-potentiating activity from Zanthoxylum bungeanum. Bioorg. Med. Chem. Lett. 2016, 26, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, I.; Takeya, K.; Itokawa, H. Distribution of unsaturated aliphatic acid amides in Japanese Zanthoxylum species. Phytochemistry 1982, 21, 1295–1298. [Google Scholar] [CrossRef]
- Koo, J.Y.; Jang, Y.; Cho, H.; Lee, C.H.; Jang, K.H.; Chang, Y.H.; Shin, J.; Oh, U. Hydroxy-α-sanshool activates TRPV1 and TRPA1 in sensory neurons. Eur. J. Neurosci. 2007, 26, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Bryant, B.P.; Mezine, I. Alkylamides that produce tingling paresthesia activate tactile and thermal trigeminal neurons. Brain Res. 1999, 842, 452–460. [Google Scholar] [CrossRef]
- Galopin, C.C.; Furrer, S.M.; Goeke, A. Pungent and tingling compounds in Asian cuisine. ACS Symp. Ser. 2004, 867, 139–152. [Google Scholar] [CrossRef]
- Mizutani, K.; Fukunaga, Y.; Tanaka, O.; Takasugi, N.; Saruwatari, Y.; Fuwa, T.; Yamauchi, T.; Wang, J.; Jia, M.R.; Li, F.Y.; et al. Amides from huajiao, pericarps of Zanthoxylum bungeanum Maxim. Chem. Pharm. Bull. 1988, 36, 2362–2365. [Google Scholar] [CrossRef]
- Ren, L.J.; Xie, F.Z. Study on alkaloids from the roots of Zanthoxylum bungeanum Maxim. Acta Pharm. Sin. 1981, 16, 006. (In Chinese) [Google Scholar] [CrossRef]
- Chen, S.Z. Study on the chemical composition of pericarps of Zanthoxylum bungeanum. J. Nanjing Univ. Tradit. Chin. Med. 1984, 16, 2–4. (In Chinese) [Google Scholar] [CrossRef]
- Kashiwada, Y.; Ito, C.; Katagiri, H.; Mase, I.; Komatsu, K.; Namba, T.; Ikeshiro, Y. Amides of the fruit of Zanthoxylum, spp. Phytochemistry 1997, 44, 1125–1127. [Google Scholar] [CrossRef]
- Mei, Y. Study on the Material Basis of the Numbing Compounds from Zanthoxylum bungeanum Maxim. Master’s Thesis, Southwest Jiaotong University, Chongqing, China, 2012. (In Chinese). [Google Scholar]
- Huang, S.; Zhao, L.; Zhou, X.L.; Yang, M.; Wang, C.J.; Weng, J. New alkylamides from pericarps of Zanthoxylum bungeanum. Chin. Chem. Lett. 2012, 23, 1247–1250. [Google Scholar] [CrossRef]
- Tang, M.P.; Wang, Z.X.; Zhou, Y.; Xu, W.J.; Li, S.T.; Wang, L.Y.; Wei, D.Q.; Qiao, Z.D. A novel drug candidate for Alzheimer’s disease treatment: Gx-50 derived from Zanthoxylum Bungeanum. J. Alzheimers Dis. 2013, 34, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.H.; Luo, B.; Sun, Y.N.; Kim, Y.H.; Wei, A.Z.; Gao, J.M. Isobutylhydroxyamides from Zanthoxylum bungeanum and their suppression of NO production. Molecules 2016, 21, 1416. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.M.; Li, X.Y.; Zhang, H.H.; Zhou, X.; Zhang, H.Q. Analysis of volatile compounds in the pericarp of Zanthoxylum bungeanum Maxim. by ultrasonic nebulization extraction coupled with headspace single-drop Microextraction and GC–MS. Chromatographia 2010, 71, 455–459. [Google Scholar] [CrossRef]
- Li, X.D.; Xue, H.L. Antifungal activity of the essential oil of Zanthoxylum bungeanum, and its major constituent on Fusarium sulphureum, and dry rot of potato tubers. Phytoparasitica 2014, 42, 509–517. [Google Scholar] [CrossRef]
- Zhang, W.J.; Guo, S.S.; You, C.X.; Geng, Z.F.; Liang, J.Y.; Deng, Z.W.; Wang, C.F.; Du, S.S.; Wang, Y.Y. Chemical composition of essential oils from Zanthoxylum bungeanum Maxim. and their bioactivities against Lasioderma serricorne. J. Oleo Sci. 2016, 65, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Z.; Zhao, Y.Q.; Li, R. Research on the chemical compounds isolated from Z. bungeanum seeds. J. Shenyang Pharm. Univ. 2006, 23, 91–92. (In Chinese) [Google Scholar] [CrossRef]
- Gong, Y.W.; Huang, Y.F.; Zhou, L.G.; Shi, X.Y.; Guo, Z.J.; Wang, M.G.; Jiang, W.B. Chemical composition and antifungal activity of the fruit oil of Zanthoxylum bungeanum Maxim. (Rutaceae) from China. J. Essent. Oil Res. 2009, 21, 174–178. [Google Scholar] [CrossRef]
- Zhu, R.X.; Zhong, K.; Zeng, W.C.; He, X.Y.; Gu, X.Q.; Zhao, Z.F.; Gao, H. Essential oil composition and antibacterial activity of Zanthoxylum bungeanum. Afr. J. Microbiol. Res. 2011, 5, 4631–4637. [Google Scholar] [CrossRef]
- Lan, Y.; Li, H.; Chen, Y.Y.; Zhang, Y.W.; Liu, N.; Zhang, Q.; Wu, Q. Essential oil from Zanthoxylum bungeanum Maxim. and its main components used as transdermal penetration enhancers: A comparative study. J. Zhejiang Univ. Sci. 2014, 15, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.B.; Dawen, S.; Mizuno, M. Flavonol glucosides in pericarps of Zanthoxylum bungeanum. Phytochemistry 1995, 39, 723–725. [Google Scholar] [CrossRef]
- Wang, W.Z.; Zhao, Y.Y.; Li, R.; Zhao, Y.Q. Study on the chemical compounds of seeds isolated from Z. bungeanum. Chin. Tradit. Herb. Drugs 2008, 39, 184–186. (In Chinese) [Google Scholar] [CrossRef]
- Tang, W.Z.; Xie, Q.M.; Guan, J.; Jin, S.H.; Zhao, Y.Q. Phytochemical profiles and biological activity evaluation of Zanthoxylum bungeanum Maxim. seed against asthma in murine models. J. Ethnopharmacol. 2014, 152, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.H.; Li, M.L. Ingredient analysis of prickly ash oil. Acta Agric. Boreali-occident. Sin. 2002, 11, 43–45. (In Chinese) [Google Scholar]
- Zhang, J.W.; Zhao, L.; Shi, B.L.; Huang, S.; Shan, L.H.; Zhou, X.L. Study on the chemical constituents of pericarps of Zanthoxylum bungeanum. West China J. Pharm. Sci. 2016, 31, 109–112. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, M.F.; Fan, R.P.; Guo, H.R.; Li, R.M.; Li, S.L. Study on gastric emptying of mice and small intestinal movement of isolated rabbits of warming herbs. J. Tradit. Chin. Med. 1984, 12, 65–67. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, M.F. Effects on warming middle burner of Zanthoxylum bungeanum Maxim. Northwest Pharm. J. 1995, 10, 89–91. (In Chinese) [Google Scholar]
- Zhang, M.F.; Shen, Y.Q.; Zhu, Z.P.; Chen, G.J. Study on warming middle and relieving pain of Zanthoxylum bungeanum Maxim. J. Chin. Mater. Med. 1991, 16, 483–497. (In Chinese) [Google Scholar]
- Yuan, T.N.; Zheng, S.L.; Shen, J. Effects of Zanthoxylum bungeanum Maxim. on the isolated duodenal smooth muscle in rabbits. Chin. Foreign Health Abstr. 2009, 6, 221–222. (In Chinese) [Google Scholar]
- Yuan, T.N. Effects of Zanthoxylum bungeanum Maxim. essential oil on the contraction of isolated colonic smooth muscle in rabbit. J. Hubei Univ. Nat. 2009, 26, 14–19. (In Chinese) [Google Scholar]
- Zhang, Z.C.; Liu, J.X.; Shen, P.; Cao, Y.G.; Lu, X.J.; Gao, X.J.; Fu, Y.H.; Liu, B.; Zhang, N.S. Zanthoxylum bungeanum pericarp extract prevents dextran sulfate sodium-induced experimental colitis in mice via the regulation of TLR4 and TLR4-related signaling pathways. Int. Immunol. Pharmacol. 2016, 41, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Omiya, Y.; Hira, Y.; Kaneko, A.; Chiba, S.; Suzuki, T.; Noguchi, M.; Watanabe, T. Daikenchuto (TU-100) ameliorates colon microvascular dysfunction via endogenous adrenomedullin in Crohn’s disease rat model. J. Gastroenterol. 2011, 46, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Ohtake, N.; Ohbuchi, K.; Mase, A.; Imamura, S.; Sudo, Y.; Miyano, K.; Yamamoto, M.; Kono, T.; Uezono, Y. Hydroxy-α-sanshool induces colonic motor activity in rat proximal colon: A possible involvement of KCNK9. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.F.; Wu, M. Study on isolated nerve of the toad of Zanthoxylum bungeanum Maxim. Guizhou Med. J. 1984, 8, 13–14. (In Chinese) [Google Scholar]
- Sun, H.F.; Wu, M.; Wang, D.M.; Liu, G. Study on blocking nerve impulsion of Zanthoxylum bungeanum Maxim. Guizhou Med. J. 1990, 14, 271–272. (In Chinese) [Google Scholar]
- Wu, Y.; Xiang, X.X. Antidepressant effect of acute administration with polyphenol extract from Zanthoxylum bungeanum Maxim. and its possible mechanism. Strait Pharm. J. 2010, 22, 33–36. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Li, L.F. The Antidepressant Effects of ZPPC in Chronically Stress Menopause Depression Mice. Master’s Thesis, Wenzhou Medical University, Wenzhou, China, 2014. (In Chinese). [Google Scholar]
- Zhu, P.H. Influence of polyphenol extract from Zanthoxylum Bungeanum Maxim. on norepinephrine, 5-hydroxytryptamine and monoamine oxidase in brain tissue of rats with post-stroke depression. J. Tradit. Chin. Med. Comb. West. Med. Zhejiang 2014, 24, 393–395. (In Chinese) [Google Scholar]
- Zhou, J.; Mu, H.O.; Wang, W. Analysis of antidepressant effects and possible mechanisms of ZPPC in chronically stressed rats. Pharm. Res. 2011, 20, 8–9. (In Chinese) [Google Scholar] [CrossRef]
- Chen, S.F.; Li, L.F.; Chen, J.; Wang, X.T.; Huang, H.J. The antidepressant effect of procyanidins extract from Zanthoxylum bungeanum Maxim. on ovariectomized model mice. J. Wenzhou Med. Univ. 2015, 45, 260–264. (In Chinese) [Google Scholar] [CrossRef]
- Nakamura, T.; Komai, N.; Isogami, I.; Ueno, K.; Ikegami, F.; One, K. Memory and learning-enhancing effect of Daikenchuto, a traditional Japanese herbal medicine, in mice. J. Nat. Med. 2006, 60, 64–67. [Google Scholar] [CrossRef]
- Wei, H.L. Effects of Gx-50 on Amyloid-Induced Inflammation in Primary Cultured Microglia Cells. Master’s Thesis, Shanghai Jiao Tong University, Shanghai, China, 2011. (In Chinese). [Google Scholar]
- Heng, L.; Li, C.; Jia, M.; Yao, X.J.; Mei, Q.B. Study on therapeutic effects of seeds oil of Zanthoxylum bungeanum Maxim. on experimental hyperlipidemia in rat. Med. J. Chin. PLA 2005, 30, 1012–1013. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, D.N.; Liu, Y.S.; Yang, Y.; Zhao, Y.W.; Wang, Z.L.; Yang, J.Q. Preventive and therapeutical effect of the kernel of Zanthoxylum bungeanum seed oil on experimental hyperlipidemia in rat. J. Fourth Mil. Med. Univ. 2007, 28, 411–413. (In Chinese) [Google Scholar] [CrossRef]
- Chen, G.; Gao, X.; Zhen, K.S.; Yin, Z.Y.; Zheng, X.X. Extract of Zanthoxylum bungeanum maxim. seed oil reduces hyperlipidemia in hamsters fed high-fat diet via activation of peroxisome proliferator-activated receptor γ. Trop. J. Pharm. Res. 2014, 13, 1837–1843. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, H.C.; Liu, S.P.; Yang, L.P.; Hai, X. Study of Zanthoxylum Bungeanum essential oil on isolated vasodilation effect in rats and investigation on the mechanism. Acta Chin. Med. Pharm. 2016, 44, 29–32. (In Chinese) [Google Scholar]
- Yang, Q.; Cao, W.D.; Zhou, X.X.; Cao, W.; Xie, Y.H.; Wang, S.W. Anti-thrombotic effects of α-linolenic acid isolated from Zanthoxylum bungeanum Maxim. seeds. BMC Complement. Altern. Med. 2014, 14, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.F.; Shen, Y.Q.; Zhu, Z.P.; Chen, G.J.; Duan, J.Y.; Song, Y.P. Study on warming middle burner and antidiarrheal effects of Zanthoxylum bungeanum Maxim. J. Chin. Med. Mater. 1994, 17, 37–40. (In Chinese) [Google Scholar]
- Yuan, J.L. The effects of anti-inflammatory and analgesic action of Zanthoxylum bungeanum Maxim. J. Chin. Med. Mater. 2010, 33, 794–797. (In Chinese) [Google Scholar] [CrossRef]
- Shi, X.P.; Zhang, W.M.; Zhang, M.Z.; Guan, R.Q. Analgesic, anti-inflammatory and anti-pruritic effects of the total alkaloids isolated from Zanthoxylum bungeanum Maxim. Chin. Wild Plant Resour. 2011, 30, 46–49. (In Chinese) [Google Scholar] [CrossRef]
- Tezuka, Y.; Irikawa, S.; Kaneko, T.; Banskota, A.H.; Nagaoka, T.; Xiong, Q.; Hase, K.; Kadotaet, S. Screening of Chinese herbal drug extracts for inhibitory activity on nitric oxide production and identification of an active compound of Zanthoxylum bungeanum. J. Ethnopharmacol. 2001, 77, 209–217. [Google Scholar] [CrossRef]
- Lee, H.G.; Lee, Y.K.; Lee, H.J.; Lee, B.H.; Kim, J.S. A study of analgesic effect of Zanthoxylum bungeanum Maxim. pharmacopuncture. Acupuncture 2017, 34, 61–74. [Google Scholar] [CrossRef]
- Makoto, T.; Richard, C.L.; Vilceanu, D.; Katta1, S.; Stucky, C.L.; Bautista, D.M. A ‘toothache tree’ alkylamide inhibits Aδ mechanonociceptors to alleviate mechanical pain. J. Physiol. 2013, 591, 3325–3340. [Google Scholar] [CrossRef]
- Lu, C.Q.; Lu, X.Y. Study on antioxidant and antibacterial effects of different kinds of Zanthoxylum bungeanum. J. Chin. Mater. Med. 1995, 20, 752–753. (In Chinese) [Google Scholar]
- Fan, J.H.; Xu, H.D.; Li, Y.J.; Fang, X. Ultrasonic-assisted extraction of total flavonoids from leaves of Zanthoxylum bungeanum and its antioxidation in vitro. J. Chin. Inst. Food Sci. Technol. 2010, 10, 22–27. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Y.J.; Luo, Z.W.; Wang, D.M.; He, F.Y.; Li, D.W. Phytochemical profiles and antioxidant and antimicrobial activities of the leaves of Zanthoxylum bungeanum. Sci. World J. 2014, 2014, 181072. [Google Scholar] [CrossRef]
- Li, P.Q.; Zhou, L.G.; Mou, Y.; Mao, Z.L. Extraction optimization of polysaccharide from Zanthoxylum bungeanum using RSM and its antioxidant activity. Int. J. Biol. Macromol. 2015, 72, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wang, H.; Wang, L.; Chai, L.Q.; Tian, C.R. Nutritional evaluation and functional properties of the antioxidant polypeptide from Zanthoxylum bungeanum Maxim. seeds kernel protein hydrolysate. CyTA J. Food 2017, 15, 325–432. [Google Scholar] [CrossRef]
- Li, J.K.; Wang, F.L.; Li, S.; Peng, Z.Q. Effects of pepper (Zanthoxylum bungeanum, Maxim.) leaf extract on the antioxidant enzyme activities of salted silver carp (Hypophthalmichthys molitrix) during processing. J. Funct. Foods 2015, 18, 1179–1190. [Google Scholar] [CrossRef]
- Yuan, T.N.; Wang, Y.L.; Wang, J.Z. Primary study of the anti-tumor effects and its mechanism of Zanthoxylum bungeanum in vivo and vitro. LiShiZhen Med. Mater. Med. Res. 2008, 19, 2915–2916. (In Chinese) [Google Scholar] [CrossRef]
- Han, S.N.; Li, Y.; Zhang, X.H.; Jiang, J.N. Extraction and anti-tumor activity of essential oil from Zanthoxylum bungeanum seeds. J. Food Sci. 2014, 35, 13–16. (In Chinese) [Google Scholar] [CrossRef]
- Huang, H.C.; Wang, R.Y.; Zhou, W.M. Anti-tumor effects of Z. bungeanum Maxim. on pheochromocytoma cells. Heilongjiang Med. J. 2010, 23, 514–515. (In Chinese) [Google Scholar] [CrossRef]
- Li, K.Y.; Zhou, R.; Jia, W.W.; Li, Z.; Li, J.Z.; Zhang, P.F.; Xiao, T.C. Zanthoxylum bungeanum essential oil induces apoptosis of HaCaT human keratinocytes. J. Ethnopharmacol. 2016, 186, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M. Research on Physiological Functions and Mechanism of Sanshool from Zanthoxylum in HepG2 Cell. Master’s Thesis, Southwest University, Chongqing, China, 2014. (In Chinese). [Google Scholar]
- You, Y.M.; Zhou, M.; Lu, H.J.; Shirima, G.G.; Cheng, Y.J.; Liu, X. Sanshool from Zanthoxylum, L. induces apoptosis in human hepatocarcinoma HepG2 cells. Food Sci. Biotechnol. 2015, 24, 2169–2175. [Google Scholar] [CrossRef]
- Zhao, X.X.; Zhang, C.Y.; Wen, F.; Lu, Z.M. The study of apoptosis and mechanism induced by Zanthoxylum bungeanum Maxim. extracts in hepatocellular carcinoma cells. J. Mod. Oncol. 2017, 25, 14–17. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Dong, H.H.; Zhang, J.F.; Zhang, L.Y. Inhibitory effect of hyperoside isolated from Zanthoxylum bungeanum leaves on SW620 human colorectalcancer cells via induction of the p53 signaling pathwayand apoptosis. Mol. Med. Rep. 2017, 16, 1125–1132. [Google Scholar]
- Tang, Y.F.; Tang, X.H.; Zhang, M.L.; Yang, Q.Q.; Hu, T. Composition and antimicrobial activity of essential oil extracted from Zanthoxylum bungeanum Maxim. Nat. Sci. J. Xiangtan Univ. 2013, 35, 64–69. (In Chinese) [Google Scholar] [CrossRef]
- Bowers, W.S.; Ortego, F.; You, X.Q.; Evans, P.P. Insect repellents from the Chinese Prickly Ash Zanthoxylum bungeanum. J. Nat. Prod. 1993, 56, 935–938. [Google Scholar] [CrossRef]
- Guo, H.X.; Liu, Q.J.; Zhang, H.Z.; Yuan, C. Separation of insecticidal material and antimicrobial activity of essential oil from Zanthorylum bungeanum Maxim. Acta Agric. Jiangxi 2008, 20, 39–40. (In Chinese) [Google Scholar] [CrossRef]
- Kou, Y.Y. The Extraction Process and Insecticidal Activity Research of Zanthoxylum bungeanum Maxim. Master’s Thesis, Wuhan Polytechnic University, Wuhan, China, 2015. (In Chinese). [Google Scholar]
- Artaria, C.; Maramaldi, G.; Bonfigli, A.; Rigano, L.; Appendino, G. Lifting properties of the alkamide fraction from the fruit husks of Zanthoxylum bungeanum. Int. J. Cosmet. Sci. 2011, 33, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.K.; Zhou, X.M.; Wu, J.; Li, J.B.; Bai, L.Y. A novel function of sanshools: The alleviation of injury from metolachlor in rice seedlings. Pestic. Biochem. Physiol. 2014, 110, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Zhong, L.J.; Hong, Z.Y.; Li, Y.M.; Liu, X.H.; Pan, L.L.; Xin, H.; Zhu, Y.Z. The effects of Zanthoxylum bungeanum extract on lipid metabolism induced by sterols. J. Pharmacol. Sci. 2014, 127, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, Y.P. Study on toxicity and Pharmacokinetics of Zanthoxylum bungeanum and Zanthoxylum schinifolium Sieb.et Zucc. LiShiZhen Med. Mater. Med. Res. 2010, 21, 1142–1143. (In Chinese) [Google Scholar] [CrossRef]
- Fang, G.S. Pharmacokinetics Studies of Alkylamide from Zanthoxylum in Rats. Master’s Thesis, Xinan University, Chongqing, China, 2014. (In Chinese). [Google Scholar]
- Rong, R.; Cui, M.Y.; Zhang, Q.L.; Zhang, M.Y.; Yu, Y.M.; Zhou, X.Y.; Yu, Z.G.; Zhao, Y.L. Anesthetic constituents of Zanthoxylum bungeanum Maxim.: A pharmacokinetic study. J. Sep. Sci. 2016, 39, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.G.; Chen, X.J. The effects of three kinds of flavourings on viscera of mouse. J. Xianning Coll. 2003, 17, 174–176. (In Chinese) [Google Scholar] [CrossRef]
- Yuan, J.L.; He, Z.M.; Wang, S.W.; Li, Y. The cute toxicity of essential oil of Zanthoxylum bungeanum. LiShiZhen Med. Mater. Med. Res. 2010, 21, 2696–2697. (In Chinese) [Google Scholar] [CrossRef]








| Preparation Name | Main Compositions | Traditional and Clinical Usages | Ref. |
|---|---|---|---|
| Hua Zheng Hui Sheng Tables | Pericarpium Zanthoxyli, Herba Leonuri, Flos Carthami, Radix Angelicae Sinensis, Lignum Sappan, Rhizoma Chuanxion, Ginseng radix and rhizome, Lignum Dalbergiae Odoriferae | Removing blood stasis, curing blood accumulation, postpartum blood stasis | [7] |
| Wu Mei Pills | Pericarpium Zanthoxyli, Ginseng radix and rhizome, Radix Angelicae Sinensis, Rhizoma Zingiberis, Fructus Mume, Herba Asari, Rhizoma Coptidis, Cortex Phellodendri, Radix Aconiti Lateralis Preparata | Clearing the liver, regulating the middle burner, clearing the upper burner, warming the lower burner, and curing ascariasis, chronic dysentery, Jue Yin headache | [7] |
| Quan Lu Pills | Pericarpium Zanthoxyli, Cornu Cervi Pantotrichum, Herba Cynomorii, Radix Bupleuri, Radix Rehmanniae, Radix Achyranthis Bidentatae, Radix Rehmaniae Praeparata, Myrrha, Semen Cuscutae, Fructus Lycii | Invigorating the kidney and essence replenishment, invigorating the spleen and supplementing Qi, and curing weak waist, cold chills, deafness, tinnitus | [7] |
| Bo Yun Tui Yi Pills | Pericarpium Zanthoxyli, Flos Buddlejae, Fructus Tribuli, Flos Chrysanthemi, Herba Equiseti Hiemalis, Feriostracum Serpentis, Herba Schizonepetae, Fructus Viticis, Herba Menthae, Radix Angelicae Sinensis, Rhizoma Chuanxion | Cleaning heat, removing wind, and improving eyesight and curing blurred vision caused by wind-heat | [7] |
| Tong Luo Qu Tong Gao | Pericarpium Zanthoxyli, Radix Angelicae Sinensis, Rhizoma Chuanxion, Flos Carthami, Fructus Piperis, Flos Caryophylli, Cortex Cinnamomi, Fructus Piperis Longi, Rhizoma Zingiberis, Camphora, Borneolum Syntheticum | Promoting blood circulation, removing meridian obstruction, dispelling cold, removing dampness, relieving swell and pain, and curing blood stagnation as well as cold dampness blocking in collaterals | [7] |
| Kang Fu Ruan Gao | Pericarpium Zanthoxyli, Radix Angelicae Dahuricae, Fructus Cnidii, Radix Inulae, Borneolum Syntheticum | Curing pruritus valvae, leucorrhea disease, itching | [7] |
| Zi Hua Shao Shang Ruan Gao | Pericarpium Zanthoxyli, Radix Arnebiae Seu Lithospermi, Radix Rehmanniae, Prepared Radix Rehmanniae, Borneolum Syntheticum, Rhizoma Coptidis, Radix Glycyrrhizae, Rhizoma Coptidis, Radix Angelicae Sinensis | Curing burning and scalding disease | [7] |
| Chang Chun Yao Jiu | Pericarpium Zanthoxyli, Rhizoma Atractylodis, Prepared Radix Rehmanniae, Fructus Gardeniae, Fructus Amomi Rotundus, Herba Epimedii, Radix Achyranthis Bidentatae, Psoralea corylifolia Linn, Radix Paeoniae Alba, Cortex Eucommiae | Curing backache caused by deficiency of the kidney, rheumatism, debility, and weak blood | [19] |
| Qian Zi Hong Ke Li | Pericarpium Zanthoxyli, Geranium strictipes Knuth, Herba Senecionis Scandentis, Cortex Schizophragmatis Integrifolii Radicis, Radix Myricae Rubrae | Curing dysentery, diarrhea caused by summer heat, dampness, and dyspepsia | [19] |
| Shen Rong Gu Ben Huan Shao Pills | Pericarpium Zanthoxyli, Ginseng radix andrhizome, Radix Aconiti Lateralis Preparata Cornu Cervi Pantotrichum, Cortex Cinnamomi, Semen Cuscutae, Cortex Eucommiae, Herba Epimedii, Radix Achyranthis Bidentatae, Actinolitum | Reinforcing the kidneys to strengthen Yang, strengthening tendons and bones, benefiting Qi | [19] |
| Zhuang Yuan Bu Xue Pills | Pericarpium Zanthoxyli, Radix Angelicae Sinensis, Colla Corii Asini, Radix Morindae Officinalis, leopard bone, Fructus Psoraleae, Semen Plantaginis, Halloysitum Rubrum, Radix Achyranthis Bidentatae, Cortex Lycii, Radix Rehmanniae, Cortex Eucommiae, Poria | Building fitness, calming nerves, reinforcing the stomach, and curing weak waist, fatigue, insomnia, forgetfulness, poor appetite, and loose stools | [20] |
| Hui Chun Jiu | Pericarpium Zanthoxyli, Radix Angelicae Sinensis, Rhizoma Atractylodis, Cortex Lycii, Flos Caryophylli, Cortex Eucommiae, Poria, Radix Aconiti Lateralis Preparata, Radix Glycyrrhizae, Radix Aucklandiae | Nourishing Yin, tonifying Yang, reinforcing the vital essence, benefiting Qi, nourishing the blood and curing spiritual burnout, weak waist, loss of appetite | [20] |
| Zhi Chuang Wai Xi Yao | Pericarpium Zanthoxyli, Rhizoma Coptidis, Radix Saposhnikoviae, Radix Glycyrrhizae, Natrii Sulfas, Galla Chinensis, Herba Houttuyniae | Removing poison, relieving itching, swelling and pain, and curing hemorrhoids, anal pain, swelling and stiffness | [20] |
| Ke Tong Ding | Pericarpium Zanthoxyli, Oleum Menthae, Radix and Rhizoma Litseae Cubebae, Rhizoma Curcumae, Rhizoma Alpiniae Officinarum, Herba Pogostemonis, Radix Scutellariae, Lignum Dalbergiae Odoriferae | Dispelling wind and dampness, promoting blood circulation, alleviating pain, and curing punch injury, rheumatism | [20] |
| Wen Shen Quan Lu Pills | Pericarpium Zanthoxyli, Radix Angelicae Sinensis, Rhizoma Chuanxion, Flos Carthami, Radix Morindae Officinalis, Rhizoma Atractylodis Macrocephalae, Fructus Psoraleae, Lignum Aquilariae Resinatum, Pericarpium Citri Reticulatae, Radix Achyranthis Bidentatae, Radix Codonopsis, Radix Rehmanniae, Radix Glycyrrhizae | Warming the kidneys, reinforcing Qi, nourishing blood, and curing dizziness, forgetful, tinnitus, weak waist, ennui, impotence | [20] |
| Jian Shen Quan Lu pills | Pericarpium Zanthoxyli, Radix Angelicae Sinensis, Rhizoma Atractylodis Macrocephalae, Fructus Psoraleae, Lignum Aquilariae Resinatum, Pericarpium Citri Reticulatae, Rhizoma Chuanxiong, Radix Rehmanniae, Poria, Radix Glycyrrhizae, Radix Astragali | Nourishing blood, reinforcing Qi, warming the kidneys, curing weak waist, mental exhaustion caused by blood deficiency | [20] |
| Chan Ma Zhen Tong Ding | Pericarpium Zanthoxyli, Herba Asari, notoginseng radix and rhizome, Venenum Bufonis, Semen Serychni, Fructus Evodiae, Radix Aconiti Kusnezoffii, Folium Sinapis, Fructus Gleditsiae, Camphora | Relaxing tendons, activating collaterals, promoting blood circulation, removing blood stasis, and curing paining joints, muscle injury, periathritis of the shoulder, hyperosteogeny | [21] |
| Suan Tong Pen Wu Ji | Pericarpium Zanthoxyli, Lignum Sappan, Radix Aconiti Kusnezoffii, Radix Aconiti, Radix Angelicae Pubescentis, Rhizoma and Radix Notopterygii, Fructus Liquidambaris, Fructus Chaenomelis, Rhizoma Arisaematis, Rhizoma Pinelliae | Relaxing tendons, activating collaterals, dispelling wind, alleviating pain, and curing sprain, repetitive strain injury, aching muscles | [21] |
| Zhen Tong Huo Luo Ding | Pericarpium Zanthoxyli, Radix Aconiti Kusnezoffii, Rhizoma Pinelliae Radix Aconiti, Camphora, Fructus Gardeniae, Radix and Rhizoma Rhei, Fructus Chaenomelis, Rhizoma Arisaematis, Rhizoma and Radix Notopterygii, Radix Angelicae Pubescentis | Relaxing tendons, activating collaterals, dispelling wind, alleviating pain, and curing eriathritis of the shoulder, hyperosteogeny, arthritis, cervical spondylopathy | [21] |
| An Wei Zhi Tong San | Pericarpium Zanthoxyli, Os Sepiae, Fructus Foeniculi, Concha Margaritifera Usta, Cortex Cinnamomi, Rhizoma Zingiberis, Rhizoma Kaempferiae, Radix and Rhizoma Rhei, Flos Caryophylli, Pericarpium Citri Reticulatae, Oleum Menthae, Radix Glycyrrhizae | Harmonizing the stomach, regulating Qi, relieving pain, and curing epigastric distention, soreness, reflux and acid regurgitation | [21] |
| Hui Sheng Kou Fu Ye | Pericarpium Zanthoxyli, Lignum Sappan, Herba Leonuri, Flos Carthami, Radix Angelicae Sinensis, Rhizoma Chuanxion, Hirudo, Rhizoma Sparganii, Rhizoma Anemones Raddeanae | Removing blood stasis, and curing primary liver cancer, lung cancer | [21] |
| Fu Fang Zhi Zi Qi Wu Ji | Pericarpium Zanthoxyli, Radix Sophorae Flavescentis, Fructus Gardeniae, Radix Arnebiae Seu Lithospermi, Radix Sanguisorbae, Borneolum Syntheticum, Radix and Rhizoma Rhei, Rhizoma Coptidis, Flos Sophorae, Herba Asari | Clearing heat, detoxicating, stopping bleeding, relieving swelling and pain, and curing incising wounds, acne of the superficial skin | [21] |
| Bing Zhi Shang Tong Qi Wu Ding | Pericarpium Zanthoxyli, Radix and Rhizoma Rhei, Fructus Gardeniae, Radix Rehmanniae, Lignum Dalbergiae Odoriferae, Radix Allii Tuberosi, Semen Serychni, Borneolum Syntheticum, Semen Persicae, Nodus Pini, Rhizoma Zingiberis | Clearing heat, detoxicating, cooling the blood, promoting blood circulation, and curing bruises, swelling, and pain caused by extravasated blood, as well as burns | [21] |
| Zhi Tong An Cha Ji | Pericarpium Zanthoxyli, Radix Sophorae Flavescentis, Flos Lonicerae, Radix Rumicis Nepalensis, Fructus Aurantii, Flos Sophorae | Clearing heat and wetness, cooling blood and stopping blood, and curing perianalpruritic induced by heat-dampness retention | [21] |
| Li Fu Kang Xi Ji | Pericarpium Zanthoxyli, Radix Sophorae Flavescentis, Cortex Phellodendri, Fructus Cnidii, Cortex Dictamni, Rhizoma Coptidis, Fructus Kochiae, Radix Isatidis, Radix Paeoniae Rubra, Radix Polygoni Multiflori, Rhizoma Smilacis Glabrae | Clearing heat and wetness, relieving itching, and curing leucorrhea disease, pruritus vulvae, infusorial vulvitis, bacterial vaginopathy | [21] |
| Qing Bai Jie Shen Xi Ye | Pericarpium Zanthoxyli, Radix Angelicae Sinensis, Radix Sophorae Flavescentis, Rhizoma Coptidis, Cortex Phellodendri, Fructus Cnidii, Radix Astragali, Radix Polygoni Multiflori, Fructus Kochiae, Folium Isatidis, Radix Paeoniae Rubra | Clearing heat and wetness, detoxicating, relieving itching, and curing pruritus vulvae, vulvitis, bacterial vaginopathy | [21] |
| Qu Fu Er Xiang Shuan | Pericarpium Zanthoxyli, Resina Draconis, Oblibanum, Fructus Cnidii, Alumen, Borax, Realgar | Removing putrid tissues and promoting the growth of new tissue, as well as curing cervical erosion | [21] |
| Ku Shen An Shi Jin | Pericarpium Zanthoxyli, Radix Sophorae Flavescentis, Herba Verbenae, Herba Taraxaci, Fructus Cnidii, Galla Chinensis, Radix Stemonae, Alumen | Clearing heat and wetness, relieving itching, and curing pruritus vulvae in females, scrotal eczema in males | [21] |
| Ri Shu An Xi Ye | Pericarpium Zanthoxyli, Radix Sophorae Flavescentis, Herba Verbenae, Herba Taraxaci, Fructus Cnidii, Galla Chinensis, Radix Stemonae, Alumen | Clearing heat and dampness, detoxicating, relieving itching, and curing pruritus vulvae in females, scrotal eczema in males | [21] |
| No. | Name | Part of Plant | Ref. |
|---|---|---|---|
| 1 | Hydroxy-α-sanshool | Pericarps | [8] |
| 2 | α-Sanshool | Pericarps | [34] |
| 3 | Hydroxy-β-sanshool | Pericarps | [8] |
| 4 | β-Sanshool | Pericarps | [34] |
| 5 | Hydroxy-γ-sanshool | Pericarps | [8] |
| 6 | γ-Sanshool | Pericarps | [8] |
| 7 | (2E,4E)-2′-Hydroxy-N-isobutyl-2,4-tetradecadienamide | Pericarps | [8] |
| 8 | (2E,4E, 8Z)-2′-Hydroxy-N-isobutyl-2,4,8-tetradecatrienamide | Pericarps | [8] |
| 9 | (2E,4E,8Z,10E,12E)-l′-Isopropenyl-N-(2′-bisobutenyl)-2,4,8,10,12-tetradecapentaenamide | Pericarps | [8] |
| 10 | (2E,4E,8Z,11E)-2′-Hydroxy-N-isobutyl-2,4,8,11-tetradecatetraenamide | Pericarps | [8] |
| 11 | (2E,7E,9E)-N-(2-Hydroxy-2-methylpropyl)-6-ethoxy-11-hydroxy-dodeca-2,7,9-trienamide | Pericarps | [26] |
| 12 | (2E,7E,9E)-N-(2-Hydroxy-2-methylpropyl)-11-ethoxy-6-hydroxy-dodeca-2,7,9-trienamide | Pericarps | [26] |
| 13 | (2E,6E,8E)-N-(2-Hydroxy-2-methylpropyl)-10-hydroxy-5-oxo-undeca-2,6,8-trienamide | Pericarps | [26] |
| 14 | (2E,4E,8E,10E,12E)-2′-Hydroxy-N-isobutyl-2,4,8,10,12-tetradecatetraenamide | Pericarps | [31] |
| 15 | (2E,4E,8E,10E,12E)-N-Isobutyl-2,4,8,10,12-tetradecapentaenamide | Pericarps | [34] |
| 16 | (2E,4E,8Z,11Z)-N-(2-Hydroxy-2-methylpropyl)-2,4,8,11-tetradeeatetraenamide | Pericarps | [35] |
| 17 | (2E,6E,8E)-N-(2-Hydroxy-2-methylpropyl)-10-oxo-2,6,8-decatrienamide | Pericarps | [35] |
| 18 | 2′-Hydroxy-N-isobytyl-[trans-2,6,8,10] dodecatetraenamide | Pericarps | [34] |
| 19 | (6RS)-(2E,7E,9E)-6-Hydroxy-N-(2-hydroxy-2-methylpropyl)-11-oxo-2,7,9-dodecatrienamide | Pericarps | [36] |
| 20 | N-[2-(3,4-Dimethoxyphenyl)ethyl]-3-phenyl-acrylamide | Pericarps | [37] |
| 21 | Bugeanumamide A | Pericarps | [38] |
| 22 | (11RS)-(2E,7E,9E)-11-Hydroxy-N-(2-hydroxy-2-methylpropyl)-6-oxo-2,7,9-dodecatrienamide | Pericarps | [38] |
| 23 | (10RS,11RS)-(2E,6Z,8E)-10,11-Dihydroxy-N-(2-hydroxy-2-methylpropyl)-2,6,8-dodecatrienamide | Pericarps | [38] |
| 24 | (6RS,11RS)-(2E,7E,9E)-N-(2-Hydroxy-2-methylpropyl)-6,11-dioxo-2,7,9-dodecatrienamide | Pericarps | [38] |
| 25 | (2E,4E,9E,11E)-N-(2-Hydroxy-2-methypropyl)-8-hydroxy-13-oxo-2,4,9,11-tetradecatetraenamide | Pericarps | [38] |
| 26 | (2E,4E,9E,11E)-N-(Hydroxy-2-methypropyl)-8,13-dihydroxy-2,4,9,11-tetradecatetraenamide | Pericarps | [38] |
| 27 | (2E)-6,6-Dimethoxy-N-(2-hydroxy-2-methylpropyl)-2-hexenamide | Pericarps | [37] |
| 28 | Zanthobungeanine | Roots | [32] |
| 29 | Demethoxy chelerythrine | Roots | [32] |
| 30 | 11-Demethoxy chelerythrine | Roots | [32] |
| 31 | l-N-Acetylanonanine | Roots | [32] |
| 32 | Arnothianamide | Roots | [32] |
| 33 | Skimmianine | Pericarps | [32] |
| 34 | Haplopine | Pericarps | [33] |
| 35 | Kokusaginine | Pericarps | [33] |
| No. | Name | Part of Plant | Ref. |
|---|---|---|---|
| 36 | Linalool | Pericarps | [9] |
| 37 | Limonene | Pericarps | [9] |
| 38 | Geraniol | Pericarps | [9] |
| 30 | p-Mentha-1,3,8-triene | Pericarps | [9] |
| 40 | Citronellal | Pericarps | [9] |
| 41 | Isopulegol | Pericarps | [9] |
| 42 | Hotrienol | Pericarps | [9] |
| 43 | 4-Terpinenyl acetate | Pericarps | [9] |
| 44 | cis-p-2-Menthen-1-ol | Pericarps | [9] |
| 45 | cis-p-Mentha-2,8-dien-1-ol | Pericarps | [9] |
| 46 | Citronellyl acetate | Pericarps | [9] |
| 47 | trans-p-Mentha-2,8-dienol | Pericarps | [9] |
| 48 | p-Mentha-1,8-dien-4-ol | Pericarps | [9] |
| 49 | Cryptone | Pericarps | [9] |
| 50 | trans-Piperitol | Pericarps | [9] |
| 51 | cis-Carveyl acetate | Pericarps | [9] |
| 52 | p-Menth-1-en-9-al | Pericarps | [9] |
| 53 | trans-Carveol | Pericarps | [9] |
| 54 | p-Mentha-1,8(10)-dien-9-ol | Pericarps | [9] |
| 55 | Isopiperitenone | Pericarps | [9] |
| 56 | p-1,8-Menthadienyl-7 acetate | Pericarps | [9] |
| 57 | 2,3-Dehydro-1,8-cineole | Pericarps | [9] |
| 58 | trans-Sabinene hydrate | Pericarps | [9] |
| 59 | trans-Sabinene hydrate acetate | Pericarps | [9] |
| 60 | Pinocarvone | Pericarps | [9] |
| 61 | Bornyl acetate | Pericarps | [9] |
| 62 | Myrtenal | Pericarps | [9] |
| 63 | trans-Pinocarveol | Pericarps | [9] |
| 64 | Myrtenol | Pericarps | [9] |
| 65 | α-Cubebene | Pericarps | [9] |
| 66 | α-Bergamotene | Pericarps | [9] |
| 67 | Germacrene B | Pericarps | [9] |
| 68 | γ-Cadinene | Pericarps | [9] |
| 69 | α-Calacorene | Pericarps | [9] |
| 70 | β-Terpineol | Pericarps | [39] |
| 71 | α-Terpineol | Pericarps | [40] |
| 72 | α-Terpinene | Pericarps | [40] |
| 73 | p-Cymene | Pericarps | [40] |
| 74 | Neryl acetate | Pericarps | [9] |
| 75 | Geranyl acetate | Pericarps | [9] |
| 76 | Carvone | Pericarps | [9] |
| 77 | β-Thujone | Pericarps | [9] |
| 78 | β-Myrcene | pericarps | [9] |
| 79 | cis-Carveol | Pericarps | [9] |
| 80 | 4-Isopropyl-l-methyl-2-cyclohexen-l-ol | Seeds | [9] |
| 81 | Linalyl anthranilate | Pericarps | [41] |
| 82 | Caryophyllene oxide | pericarps | [41] |
| 83 | Germacrene D | Pericarps | [41] |
| 84 | Nerol | Pericarps | [41] |
| 85 | Eucalyptol | Pericarps | [41] |
| 86 | 24-en-Cycloartenone | Seeds | [42] |
| 87 | Camphene | Pericarps | [43] |
| 88 | β-Caryophyllene | Pericarps | [43] |
| 89 | α-Cadinol | Pericarps | [43] |
| 90 | β-Elemene | Pericarps | [43] |
| 91 | Myrcene | Pericarps | [43] |
| 92 | Carvacrol | Pericarps | [43] |
| 93 | (E)-β-Ocimene | Pericarps | [43] |
| 94 | (Z)-β-Ocimene | Pericarps | [43] |
| 95 | Sabinene | Pericarps | [43] |
| 96 | α-Terpinyl acetate | Pericarps | [43] |
| 97 | Piperitone | Pericarps | [43] |
| 98 | α-Thujene | Pericarps | [43] |
| 99 | β-Pinene | Pericarps | [44] |
| 100 | β-Phellandrene | Pericarps | [45] |
| 101 | γ-Terpinene | Pericarps | [45] |
| 102 | α-Pinene | pericarps | [45] |
| 103 | Terpinolene | Pericarps | [45] |
| No. | Name | Part of Plant | Ref. |
|---|---|---|---|
| 104 | Rutin | Leaves | [10] |
| 105 | Syringetin-3-glucoside | Leaves | [10] |
| 106 | Isorhamnetin-3-glucoside | Leaves | [10] |
| 107 | Quercetin 3-arabinoside | Pericarps | [10] |
| 108 | 3,5,7,3′,4′-Pentahydroxyflavone | Leaves | [23] |
| 109 | Quercetin 3-O-α-l-rhamnoside | Leaves | [23] |
| 110 | Quercetin 3-O-β-d-glucoside | Leaves | [23] |
| 111 | Trifolin | Leaves | [23] |
| 112 | Quercetin 3-O-β-d-galactoside | Leaves | [23] |
| 113 | Kaempferol 3-O-α-l-rhamnoside | Leaves | [23] |
| 114 | Isorhamnetin 3-O-α-l-rhamnoside | Leaves | [23] |
| 115 | Kaempferol-7-rhamnoside | Leaves | [10] |
| 116 | Apigenin-8-C-glucoside | Leaves | [10] |
| 117 | Apigenin-8-C-arabinoside | Leaves | [10] |
| 118 | Quercetin-3-rutinoside-7-rhamnoside | Leaves | [10] |
| 119 | Kaempferol-3-rutinoside | Leaves | [10] |
| 120 | Quercetin 3′,4′-dimethyl ether 7-glucoside | Pericarps | [46] |
| 121 | Tamarixetin 3,7-bis-glucoside | Pericarps | [46] |
| 122 | Isorhamnetin 7-glucoside | Pericarps | [46] |
| 123 | 3,5,6-Trihydroxy-7,4′-dimethoxy flavone | Pericarps | [46] |
| 124 | 5-Feruloyquinic acid | Leaves | [10] |
| 125 | Chlorogenic acid | Leaves | [10] |
| 126 | Sitosterol β-glucoside | Pericarps | [46] |
| 127 | l-sesamin | Pericarps | [46] |
| 128 | Quinic acid | Leaves | [10] |
| 129 | Epicatechin | Leaves | [10] |
| No. | Name | Part of Plant | Ref. |
|---|---|---|---|
| 130 | Nonanoic acid | Pericarps | [49] |
| 131 | Tetradecanoic acid | Seeds | [11] |
| 132 | Pentadecanoic acid | Seeds | [11] |
| 133 | Hexadecanoic acid | Seeds | [11] |
| 134 | Stearic acid | Seeds | [11] |
| 135 | Eicosoic acid | Seeds | [11] |
| 136 | Oleic acid | Seeds | [11] |
| 137 | Palmitoleic acid | Seeds | [49] |
| 138 | Linolenic acid | Seeds | [48] |
| 139 | Linoleic acid | Seeds | [48] |
| No. | Name | Part of Plant | Ref. |
|---|---|---|---|
| 140 | Rosefuran | Pericarps | [9] |
| 141 | Myrcene epoxide | Pericarps | [9] |
| 142 | Perillene | Pericarps | [9] |
| 143 | Vanillic acid-4-glucoside | Leaves | [10] |
| 144 | β-Sitosterol | Roots | [32] |
| 145 | Daucosterol | Seeds | [47] |
| 146 | Isoimperatorin | Seeds | [47] |
| 147 | Methyl-4-hydroxyphenylacrylate | Pericarps | [50] |
| 148 | 7-Methoxycoumarin | Pericarps | [50] |
| 149 | Xanthoxylin | Pericarps | [50] |
| Pharmacological Effects | Detail | Extracts/Compounds | Minimal Active Concentration/Dose | In Vitro/In Vivo | Ref. |
|---|---|---|---|---|---|
| Regulation on gastrointestinal smooth muscle | WEZB | 4.0 and 12 mg/mL (i.g.) | in vivo | [51,52] | |
| Effect on the digestive system | Anti-ulcer effects | Water extracts of Z. bungeanum (WEZB) | 2.5, 5, and 10 g/kg (i.g.(intragastric administration), crude herb mass equivalent) | in vivo | [53] |
| Anti-diarrhea effects | PEZB | 3.0 and 6.0 mL/kg (i.g.) | in vivo | [53] | |
| WEZB | 5 and 10 g/kg (i.g., crude herb mass equivalent) | in vivo | [53] | ||
| Inhibiting contraction of isolated duodenal smooth muscle | EOZB | 0.1 mg/mL | in vitro | [54] | |
| Inhibiting contraction of isolated colon smooth muscle | EOZB | 0.4 g/L (i.g.) | in vivo | [55] | |
| Alleviating DSS-induced experimental colitis | WEZB | 0.5,1.0, and 2.0 g/kg (i.g., for 14 days) | in vivo | [56] | |
| Accelerating defecation | Hydroxy-α-sanshool (HAS) | 50 mg/kg (per os (p.o.), crude herb mass equivalent) | in vivo | [57] | |
| Improving blood flow of the colon | HAS | 0.3 mg/kg | in vivo | [57] | |
| Improving release of ADM from intestinal epithelial cells | HAS | 0.3, 10, and 30 μmol/L | in vitro | [57] | |
| Enhancing long distance contraction of the proximal colon | HAS | 3, 10, and 30 μM | in vitro | [58] | |
| Effect on the nervous system | Blocking nerve impulse | Essential oils of Z. bungeanum (EOZB) and WEZB | 20% | in vitro | [59,60] |
| Anti-depressive effects on behavioral despair models | PEZB | 50 mg/kg (i.g.) | in vivo | [61] | |
| Reducing time of tail suspension | PEZB | 50 mg/kg (i.g., for 21 days) | in vivo | [62] | |
| Upregulate NE and 5-HT | PEZB | 50 mg/kg (i.g., for 21 days) | in vivo | [63] | |
| Anti-depressive effects in the unpredictable stress model and ovariectomized model | PEZB | 50 mg/kg (i.g., for 21 days) | in vivo | [64,65] | |
| Shorten the escape latency in mice | HAS | 5 mg/kg (p.o.) | in vitro | [66] | |
| Inhibiting Aβ-induced neuronal apoptosis and reducing neuronal toxicity | gx-50 | 5 μM | in vitro | [37] | |
| Enhancing the cross-platform times | gx-50 | 1 mg/kg(i.p., for 2 months) | in vivo | [37] | |
| Inhibiting cytokine release | gx-50 | 500 μM | in vitro | [67] | |
| Enhancing neurite outgrowth | Z. bungeanum (ZP)-amide A, ZP-amide B, ZP-amide C | 20 μM | in vitro | [26] | |
| Effect on the circulatory system | Reducing CHOL, TG, LDL, increasing HDL-C | Seed oil of Z. bungeanum (SOZB) | 5, 10, and 20 mL/kg (i.g., for 4 weeks) | in vivo | [68] |
| Reducing HBV, HLV, CHOL, TG and increasing HDL-C | SOZB | 2.5 mL/kg (i.g., for 10 weeks) | in vivo | [69] | |
| Reducing TG, TC, LDL-C, MDA, and NO | SOZB | 2.5, 5, and 10 g/kg (i.g., for 30 days) | in vivo | [70] | |
| Relaxing contracted aortic muscle | EOZB | 2.0, 4.0, 6.0, 8.0, and 10.0 μL/mL | In vitro | [71] | |
| Increased the survival rate of mice subjected to collagen-adrenaline | (Alpha-linolenic acid) ALA | 250 mg/kg (p.o., for 10 days) | in vivo | [72] | |
| Prolonged hemorrhage and coagulation time | ALA and its mixture | 50, 100, and 250 mg/kg (p.o., for 10 days) | in vivo | [72] | |
| Decreased platelet aggregation | ALA and its mixture | 70 and 175 mg/kg (p.o., for 10 days) | in vivo | [72] | |
| Anti-inflammatory and analgesic effects | Inhibiting dimethylbenzene-induced ear oedema, carrageenan-induced rat paw oedema and acetic acid-induced torsion | WEZB | 2.5, 5.0, and 10 g/kg (i.g., for 3 days, crude herb mass equivalent) | in vivo | [73] |
| DEZB | 1.5, 3.0, and 6.0 mL/kg (i.g.) | in vivo | [73] | ||
| Inhibiting dimethylbenzene-induced oedema ear, acetic acid-induced pain | EOZB | 0.05, 0.1, and 0.2 g/kg (i.g., for 14 days) | in vivo | [74] | |
| Inhibiting hot-plate-induced pain and dextran-40-induced itch–scratch responses | Alkaloids of Z. bungeanum (AZB) | 118, 236, and 472 mg/kg (i.g., for 3 days) | in vivo | [75] | |
| Inhibiting NO production | MEZB | 200 μM | in vitro | [76] | |
| Inhibiting iNOS mRNA expression | 4-O-β-d-Glucopyranosyldihydroferulic acid | IC50 = 6.5 μg/mL | in vitro | [76] | |
| Analgesic effect on formalin test | EEZB, MEZB | 40 μL, 5% (i.p.) | in vivo | [77] | |
| Relieving pain on tail-flick test | EEZB | 20 μL, 5% (i.p.) | in vivo | [77] | |
| Inhibiting the excitability of Aδ mechanosensory nociceptors | HAS | IC50 = 70 ± 7 μM | in vitro | [78] | |
| Inhibiting effects on nitric oxide (NO) production in LPS-stimulated RAW 264.7 macrophages | ZP-amide D, ZP-amide E, ZP-amide F and ZP-amide G | IC50 = 48.7 ± 0.32, 27.1 ± 1.15, 49.8 ± 0.38, and 39.4 ± 0.63 μM, respectively | in vitro | [38] | |
| Antioxidant effect | Reducing MDA | WEZB | 0.0195, 0.039, and 0.156 mg/mL | in vitro | [79] |
| Scavenging DPPH radicals | FWEZBL, FEEZBL and FAEZBL | IC50 = 24, 17.5, and 7.6 μg/mL, respectively | in vitro | [80] | |
| SOZB | Not mentioned | in vitro | [11] | ||
| EEZBL, EAEEZBL, AEEZBL and MEEZBL | IC50 = 40.75 ± 0.21, 13.20 ± 0.85, 18.55 ± 0.35 and 85.85 ± 2.19 μg/mL, respectively | in vitro | [23,81] | ||
| Polysaccharide of Z. bungeanum (PZB) | EC50 = 0.021 mg/mL | in vitro | [82] | ||
| Three fractions (<10 kDa, 10~30 kDa, and >30 kDa) of polypeptide of Z. bungeanum seeds kernel (PZBSK) | 10 mg/mL | in vitro | [83] | ||
| Quercetin, Quercitrin, Quercetin-3-O-β-d-glucoside, Hyperoside, Rutin and Isorhamnetin 3-O-α-l-rhamnoside | IC50 = 0.009 ± 0.001, 0.011 ± 0.001, 0.012 ± 0.001, 0.011 ± 0.001, 0.016 ± 0.001, and 0.028 ± 0.001 mM, respectively | in vitro | [23] | ||
| Reducing ferric and ABTS+ radical | AEEZBL | 615.88 ± 1.86 and 2147.83 ± 23.08 μmol equiv. Trolox/g, respectively | in vitro | [23,81] | |
| Reducing Fe3+ | PZB | EC50 = 0.011 mg/mL | in vitro | [82] | |
| Reducing hydroxyl radical | PZB | EC50 = 0.008 mg/mL | in vitro | [82] | |
| Chelating Fe2+ | PZB | EC50 = 0.056 mg/mL | in vitro | [82] | |
| Decreased hexanal content, TBARS value, and LOX | EEZBL | 0.015%, 0.030%, and 0.045%, for 8 days | in vitro | [25] | |
| Increased catalase, superoxide dismutase, and glutathione peroxidase activities, decreased PV TBARS values | EEZBL | 0.018% | in vitro | [84] | |
| Chlorogenic acid, Hyperoside and Quercitrin | 0.01% | in vitro | [84] | ||
| Increased cell growth rate of E. coli | Quercetin, Quercitrin, Quercetin-3-O-β-d-glucoside, Hyperoside, rutin and Isorhamnetin 3-O-α-l-Rhamnoside | Not mentioned | in vitro | [22] | |
| Inhibitory capacity on lipid peroxidation | Vitexin, Quercitrin, Afzelin, Trifolin | IC50 = 0.014 ± 0.001 0.013 ± 0.005, 0.065 ± 0.003, and 0.040 ± 0.001 mM, respectively | in vitro | [23] | |
| Anti-tumor effect | Anti-tumor effects on H22 | EOZB | 4 mg/mL | in vitro | [85] |
| Inhibitory effects on the growth of tumor in mice | EOZB | 10, 25, 50, and 100 mg/kg | in vivo | [85] | |
| Anti-tumor effects on HeLa, A549, k562 | EOZB | IC50 = 11.2 ± 0.2, 6.26 ± 0.05 and 1.37 ± 0.03 mg/mL, respectively | in vitro | [86,87] | |
| Anti-tumor effects on PC12 | EOZB | 0.5 mg/mL~2.0 mg/mL | in vitro | [86,87] | |
| Anti-proliferative effect towards HaCaT | EOZB | IC50 = 0.024% (v/v, for 48 h) | in vitro | [88] | |
| Inhibiting PC-3 cells, HEp-2 cells, Hela cells, MFC-7 cells | EOZB | IC50 = 0.04%, 0.021%, 0.03%, and 0.023%, respectively | in vitro | [88] | |
| Anti-proliferation effects against HepG2 cells | Sanshools | 0~250 μg/mL | in vitro | [89,90] | |
| Inducting apoptosis activity against HepG2 cells | Sanshools | (0~200 μg/mL) | in vitro | [89,90] | |
| Inducing apoptosis and inhibiting cell growth in HepG2 cells | EEZB | 1 μg/mL~8 μg/mL(for 48 h) | in vitro | [91] | |
| Inhibitory effects against SW620 cell | Hyperoside | IC50 = 19.51 ± 4.95 μM for 96 h | in vitro | [92] | |
| Inhibiting proliferation of HaCaT cells | d-Limonene, Terpinen-4-ol and β-Myrcene | IC50 = 0.009%, 0.028%, 0.013% (v/v, for 48 h), respectively | in vitro | [88] | |
| Anti-bacterial and anti-fungal effects | Decreased viable count of S. aureus and E. coli | WEZB | 5 mg/mL (for 4 days, crude herb mass equivalent) | in vitro | [79] |
| Inhibitory effects against Alternariasolani, B. theobromae, F. oxysporum f.sp. cucumerinum, F. oxysporum f.sp. niverum, B. maydis, L. maculans, M. grisea, R. cerealis, R. solani, V. Pirina, and V. dahlia | EOZB | IC50 = 0.44, 0.48, 0.43, 0.48, 0.24, 0.13, 0.28, 0.27, 0.24, 0.41, and 0.32 mg/mL, respectively | in vitro | [43] | |
| Inhibiting the growth of R. solani and R. cerealis mycelia | EOZB | IC50 = 0.95 and 1.22 mg/mL, respectively | in vitro | [43] | |
| Inhibitory effects towards B. subtilis, Salmonella, S.aureus, B. cereus, E. coli, P. vulgaris, P. Crtinum Thom, A. flavus, A. niger, R. Nigricans, and S.cerevisiae | EOZB | Minimum bacteriacidal (or fungicidal) (MIC/MFC) concentrations = 25, 6.25, 25, 12.5, 12.5, 12.5, 12.5, 12.5, 12.5, 25, and 12.5 mL/L, respectively | in vitro | [93] | |
| Inhibiting food-borne bacteria S. aureus, B. subtilis, B. cereus, B. Laubach, and E. coli | EOZB | MIC = 5.0, 1.25, 2.5, 1.25, and 2.5 mg/mL, respectively. MBC = 20, 2.5, 10, 5.0, and 5.0 mg/mL, respectively | in vitro | [44] | |
| Inhibitory effects against F. sulphureum | EOZB and α-Pinene | MIC = 6.25% and 12.50% | in vitro | [40] | |
| Reducing the lesion diameter of potato inoculated with F. Sulphureum | EOZB and α-Pinene | 6.25% and 12.50% | in vivo | [40] | |
| Inhibitory activity against B. cinerea, P. oryzae, P. piricola, G. Cingulate, and V. pyrina | EEZBL | IC50 = 11.82 ± 1.15, 12.31 ± 0.45, 39.48 ± 2.25, 13.00 ± 1.34, and 33.22 ± 3.61 mg/mL, respectively | in vitro | [81] | |
| Chloroform fraction of EEZBL | IC50 = 9.39 ± 0.07, 4.18 ± 0.08, 10.89 ± 1.62, 0.83 ± 0.24, and 5.35 ± 0.34 mg/mL, respectively | in vitro | [81] | ||
| Insecticide effects | Repellent activity against ants | Piperitone, 4-Terpineol, and Linalool | Not mentioned | in vitro | [94] |
| Anti-insect effects towards T. castaneum | Petroleum ether, Dichloromethane and Diethyl ether fraction of the EOZB | LD50 = 0.0713, 0.11699, and 0.12267 μL | in vitro | [95] | |
| MEZB | 0.5, 1.0, and 1.5 mg/mL | in vitro | [96] | ||
| Anti-insect activity against aedes albopictus | EOZB | 15, 25, 35, and 45 μg/mL | in vitro | [96] | |
| Insecticidal effects against L. serricorne adults | EOZB obtained hydrodistillation and supercritical fluid CO2 | LC50 = 3.99 and 12.54 μg/mL | in vitro | [41] | |
| Anti-insect activity against L. serricorne | Eucalyptol, Limonene, γ-Terpinene, Linalool, α-Terpineol and 4-Terpinenol | LC50 = 5.18, 14.07, 12.01, 18.04, 3.27, and 6.90 mg/L, respectively | in vitro | [41] | |
| Contact toxicity against L. serricorne | Eucalyptol, Limonene, γ-Terpinene, Linalool, α-Terpineol and 4-Terpinenol | LD50 = 15.58, 13.66, 14.19, 12.74, 11.99, and 8.62 μg/adult, respectively | in vitro | [41] | |
| Other pharmacological effects | Relaxing subcutaneous muscles | Sanshools | Not mentioned | in vivo | [97] |
| Alleviating rice-seedling injury | Sanshools | 0.8 mg/mL | in vitro | [98] | |
| Prolonging the LPIA | Seeds of Z. bungeanum (SZB) | 0.25, 0.5, and 1.0 g/kg | in vitro | [48] | |
| Reduce the cough number | SZB | 0.25, 0.5, and 1.0 g/kg | in vitro | [48] | |
| Anti-fatigue and anti-anoxia ability | SZB | 0.5, 1.0, and 2.0 g/kg (i.g.) | in vitro | [48] | |
| Enhancing the percutaneous absorption | EOZB | 3% | in vitro | [45] | |
| Terpinen-4-ol,1,8-Cineole and Limonene | 3% | in vitro | [45] | ||
| Decrease serum TC and TG level | n-Butanol fraction of Z. bungeanum | 50 mg/kg and 200 mg/kg (i.g., for 4 weeks) | in vivo | [99] | |
| Decrease TC, TG, FC level, apoB secretion, and increased apoA1 | n-Butanol fraction of Z. bungeanum | 0.05, 0.1, and 0.2 mg/mL | in vitro | [99] |
| Extracts/ Compounds | Animal/ Cell Line | Minimal Toxic Concentration/Dose | Toxic Effects | Ref. |
|---|---|---|---|---|
| WEZB | Mice | LD50 = 45 g/kg (i.g., crude herbs mass equal) | Death | [16] |
| WEZB | Mice | LD50 = 51.14 g/kg (i.g., crude herbs mass equal) | Death | [100] |
| WEZB | Mice | 0.5, 1.0, 2.0 and 4.0 g/kg (i.g.) | Ballooning degeneration, cytoplasm rarefaction | [103] |
| EOZB | Mice | (LD50 = 2.27, 2.03, 4.64 and 5.32 g/kg of i.g., i.p., i.m., i.h., respectively | Death | [104] |
| EOZB | HaCaT cells and CCC-ESF-1 cells | IC50 = 2.435 mg/mL and 3.649 mg/mL, respectively | Inducing cell viability | [45] |
| WEZB | J774.1 cells | 100, 200, and 400 μg/mL (for 18 h) | Non-toxic | [56] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, J.; Zhu, L.; Li, T.; Jiang, W.; Zhou, J.; Peng, W.; Wu, C. Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology. Int. J. Mol. Sci. 2017, 18, 2172. https://doi.org/10.3390/ijms18102172
Zhang M, Wang J, Zhu L, Li T, Jiang W, Zhou J, Peng W, Wu C. Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology. International Journal of Molecular Sciences. 2017; 18(10):2172. https://doi.org/10.3390/ijms18102172
Chicago/Turabian StyleZhang, Mengmeng, Jiaolong Wang, Lei Zhu, Tao Li, Weidong Jiang, Juan Zhou, Wei Peng, and Chunjie Wu. 2017. "Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology" International Journal of Molecular Sciences 18, no. 10: 2172. https://doi.org/10.3390/ijms18102172
APA StyleZhang, M., Wang, J., Zhu, L., Li, T., Jiang, W., Zhou, J., Peng, W., & Wu, C. (2017). Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology. International Journal of Molecular Sciences, 18(10), 2172. https://doi.org/10.3390/ijms18102172