Understanding the Contribution of Zinc Transporters in the Function of the Early Secretory Pathway
Abstract
:1. Introduction
2. Brief Overview of the Properties of ZNT and ZIP Transporters
3. Regulation of Zinc Homeostasis by Zinc Transporters in the Early Secretory Pathway
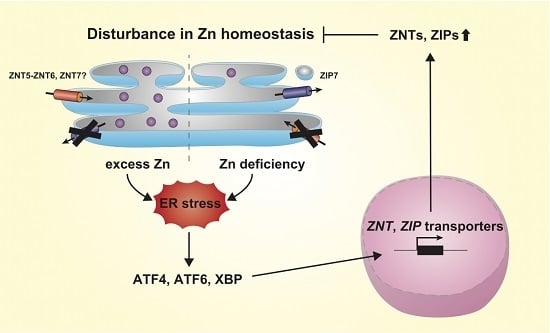
4. Regulation of Expression of ZNT and ZIP Transporters by ER Stress
5. Importance of ZNT Transporters in the Activation of Ectoenzymes in the Early Secretory Pathway
5.1. ZNT Transporters Involved in Zinc-Requiring Ectoenzyme Activation
5.2. Insight into the Activation of TNAP and Other Ectoenzymes by ZNT5-ZNT6 Heterodimers and ZNT7 Homodimers
6. Importance of ZNT Transporters in Zinc-Related Regulated Secretory Pathway: After the Early Secretory Pathway
7. Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ATX | Autotaxin |
| CAIX | Carbonic anhydrase IX |
| CDF | Cation diffusion facilitator |
| ER | Endoplasmic reticulum |
| KO | Knockout |
| MMP | Matrix metalloproteinase |
| PP | Pro-Pro |
| S(E)R | Sarco(endo)plasmic reticulum |
| SLC | Solute carrier |
| TM | Transmembrane |
| TNAP | Tissue non-specific alkaline phosphatase |
| TNZD | Transient neonatal zinc deficiency |
| UPR | Unfolded protein response |
| ZIP | Zrt- and Irt-like protein |
| ZNT | Zn transporter |
References
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Takeda, T.A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Zinc through the three domains of life. J. Proteome Res. 2006, 5, 3173–3178. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Li, Y. Coordination dynamics of zinc in proteins. Chem. Rev. 2009, 109, 4682–4707. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Devirgiliis, C.; Zalewski, P.D.; Perozzi, G.; Murgia, C. Zinc fluxes and zinc transporter genes in chronic diseases. Mutat. Res. 2007, 622, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report No. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar]
- Kambe, T.; Hashimoto, A.; Fujimoto, S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell. Mol. Life Sci. 2014, 71, 3281–3295. [Google Scholar] [CrossRef] [PubMed]
- Lichten, L.A.; Cousins, R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.L.; McCormick, N.H.; Velasquez, V.; Lopez, V. Zinc in specialized secretory tissues: Roles in the pancreas, prostate, and mammary gland. Adv. Nutr. 2011, 2, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, S.R.; Kelleher, S.L. Zinc networks: The cell-specific compartmentalization of zinc for specialized functions. Biol. Chem. 2012, 393, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T. An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway. Biosci. Biotechnol. Biochem. 2011, 75, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T. Regulation of zinc transport. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Culotta, V., Scott, R.A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 301–309. [Google Scholar] [CrossRef]
- Kambe, T. Molecular Architecture and Function of ZnT Transporters. Curr. Top. Membr. 2012, 69, 199–220. [Google Scholar] [PubMed]
- Lu, M.; Fu, D. Structure of the zinc transporter YiiP. Science 2007, 317, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chai, J.; Fu, D. Structural basis for autoregulation of the zinc transporter YiiP. Nat. Struct. Mol. Biol. 2009, 16, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Coudray, N.; Valvo, S.; Hu, M.; Lasala, R.; Kim, C.; Vink, M.; Zhou, M.; Provasi, D.; Filizola, M.; Tao, J.; et al. Inward-facing conformation of the zinc transporter YiiP revealed by cryoelectron microscopy. Proc. Natl. Acad. Sci. USA 2013, 110, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chai, J.; Cheng, J.; D’Mello, R.; Chance, M.R.; Fu, D. Visualizing the kinetic power stroke that drives proton-coupled zinc(II) transport. Nature 2014, 512, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Ohana, E.; Hoch, E.; Keasar, C.; Kambe, T.; Yifrach, O.; Hershfinkel, M.; Sekler, I. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J. Biol. Chem. 2009, 284, 17677–17686. [Google Scholar] [CrossRef] [PubMed]
- Shusterman, E.; Beharier, O.; Shiri, L.; Zarivach, R.; Etzion, Y.; Campbell, C.R.; Lee, I.H.; Okabayashi, K.; Dinudom, A.; Cook, D.I.; et al. ZnT-1 extrudes zinc from mammalian cells functioning as a Zn(2+)/H(+) exchanger. Metallomics 2014, 6, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Fukunaka, A.; Suzuki, T.; Kurokawa, Y.; Yamazaki, T.; Fujiwara, N.; Ishihara, K.; Migaki, H.; Okumura, K.; Masuda, S.; Yamaguchi-Iwai, Y.; et al. Demonstration and characterization of the heterodimerization of ZnT5 and ZnT6 in the early secretory pathway. J. Biol. Chem. 2009, 284, 30798–30806. [Google Scholar] [CrossRef] [PubMed]
- Golan, Y.; Berman, B.; Assaraf, Y.G. Heterodimerization, altered subcellular localization, and function of multiple zinc transporters in viable cells using bimolecular fluorescence complementation. J. Biol. Chem. 2015, 290, 9050–9063. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Feresin, R.G.; Falcon-Perez, J.M.; Salazar, G. Differential Targeting of SLC30A10/ZnT10 Heterodimers to Endolysosomal Compartments Modulates EGF-Induced MEK/ERK1/2 Activity. Traffic 2016, 17, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Kurokawa, Y.; Chiche, J.; Pouyssegur, J.; Sato, H.; Fukuzawa, H.; Nagao, M.; Kambe, T. Dissecting the Process of Activation of Cancer-promoting Zinc-requiring Ectoenzymes by Zinc Metalation Mediated by ZNT Transporters. J. Biol. Chem. 2017, 292, 2159–2173. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Itsumura, N.; Tsuji, T.; Anan, Y.; Tsuji, N.; Ogra, Y.; Kimura, T.; Miyamae, Y.; Masuda, S.; Nagao, M.; et al. Cooperative functions of ZnT1, metallothionein and ZnT4 in the cytoplasm are required for full activation of tnap in the early secretory pathway. PLoS ONE 2013, 8, e77445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golan, Y.; Itsumura, N.; Glaser, F.; Berman, B.; Kambe, T.; Assaraf, Y.G. Molecular Basis of Transient Neonatal Zinc Deficiency: Novel ZNT2 Mutations Disrupting ZINC Binding and Permeation. J. Biol. Chem. 2016, 291, 13546–13559. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Lin, W.; Chai, J.; Hershfinkel, M.; Fu, D.; Sekler, I. Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc. Natl. Acad. Sci. USA 2012, 109, 7202–7207. [Google Scholar] [CrossRef] [PubMed]
- Nishito, Y.; Tsuji, N.; Fujishiro, H.; Takeda, T.; Yamazaki, T.; Teranishi, F.; Okazaki, F.; Matsunaga, A.; Tuschl, K.; Rao, R.; et al. Direct comparison of manganese detoxification/efflux proteins and molecular characterization of ZnT10 as a manganese transporter. J. Biol. Chem. 2016, 291, 14773–14787. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Giedroc, D.P. Functional Determinants of Metal Ion Transport and Selectivity in Paralogous Cation Diffusion Facilitator Transporters CzcD and MntE in Streptococcus pneumoniae. J. Bacteriol. 2016, 198, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Montanini, B.; Blaudez, D.; Jeandroz, S.; Sanders, D.; Chalot, M. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: Improved signature and prediction of substrate specificity. BMC Genom. 2007, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Gustin, J.L.; Zanis, M.J.; Salt, D.E. Structure and evolution of the plant cation diffusion facilitator family of ion transporters. BMC Evol. Biol. 2011, 11, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambe, T.; Suzuki, T.; Nagao, M.; Yamaguchi-Iwai, Y. Sequence similarity and functional relationship among eukaryotic ZIP and CDF transporters. Genom. Proteom. Bioinform. 2006, 4, 1–9. [Google Scholar] [CrossRef]
- Antala, S.; Ovchinnikov, S.; Kamisetty, H.; Baker, D.; Dempski, R.E. Computation and Functional Studies Provide a Model for the Structure of the Zinc Transporter hZIP4. J. Biol. Chem. 2015, 290, 17796–17805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, J.; Fellner, M.; Zhang, C.; Sui, D.; Hu, J. Crystal structures of a ZIP zinc transporter reveal a binuclear metal center in the transport pathway. Sci. Adv. 2017, 3, e1700344. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Fukada, T.; Hosaka, T.; Yamasaki, S.; Ohashi, W.; Hojyo, S.; Miyai, T.; Nishida, K.; Yokoyama, S.; Hirano, T. Biochemical characterization of human ZIP13 protein: A homo-dimerized zinc transporter involved in the spondylocheiro dysplastic Ehlers-Danlos syndrome. J. Biol. Chem. 2011, 286, 40255–40265. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Muraina, I.A.; Brethour, D.; Schmitt-Ulms, G.; Nimmanon, T.; Ziliotto, S.; Kille, P.; Hogstrand, C. Zinc transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration. Biochem. J. 2016, 473, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Chai, J.; Love, J.; Fu, D. Selective electrodiffusion of zinc ions in a Zrt-, Irt-like protein, ZIPB. J. Biol. Chem. 2010, 285, 39013–39020. [Google Scholar] [CrossRef] [PubMed]
- Gaither, L.A.; Eide, D.J. Functional expression of the human hZIP2 zinc transporter. J. Biol. Chem. 2000, 275, 5560–5564. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Girijashanker, K.; Dalton, T.P.; Reed, J.; Li, H.; Soleimani, M.; Nebert, D.W. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: Characterization of transporter properties. Mol. Pharmacol. 2006, 70, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Girijashanker, K.; He, L.; Soleimani, M.; Reed, J.M.; Li, H.; Liu, Z.; Wang, B.; Dalton, T.P.; Nebert, D.W. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: Similarities to the ZIP8 transporter. Mol. Pharmacol. 2008, 73, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Kambe, T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 2011, 3, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sui, D.; Hu, J. Structural insights of ZIP4 extracellular domain critical for optimal zinc transport. Nat. Commun. 2016, 7, 11979. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, S.; Huo, H.; Salehzadeh, A.; Pocanschi, C.L.; Watts, J.C.; Wille, H.; Westaway, D.; Rogaeva, E.; St George-Hyslop, P.H.; Schmitt-Ulms, G. Family reunion—The ZIP/prion gene family. Prog. Neurobiol. 2011, 93, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Jenkitkasemwong, S.; Wang, C.Y.; Mackenzie, B.; Knutson, M.D. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 2012, 25, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Vembar, S.S.; Brodsky, J.L. One step at a time: Endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 2008, 9, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.R.; Cohen-Doyle, M.F.; Thomas, D.Y.; Williams, D.B. Localization of the lectin, ERp57 binding, and polypeptide binding sites of calnexin and calreticulin. J. Biol. Chem. 2002, 277, 29686–29697. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Ihara, Y.; Leach, M.R.; Cohen-Doyle, M.F.; Williams, D.B. Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO J. 1999, 18, 6718–6729. [Google Scholar] [CrossRef] [PubMed]
- Solovyov, A.; Gilbert, H.F. Zinc-dependent dimerization of the folding catalyst, protein disulfide isomerase. Protein Sci. 2004, 13, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.D.; Wang, F.; MacDiarmid, C.W.; Clark, S.; Lyons, T.; Eide, D.J. Zinc and the Msc2 zinc transporter protein are required for endoplasmic reticulum function. J. Cell Biol. 2004, 166, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Yamazaki, T.; Ishida, Y.; Suzuki, T.; Oda, K.; Nagao, M.; Yamaguchi-Iwai, Y.; Kambe, T. Zinc transport complexes contribute to the homeostatic maintenance of secretory pathway function in vertebrate cells. J. Biol. Chem. 2006, 281, 17743–17750. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, W.; Kimura, S.; Iwanaga, T.; Furusawa, Y.; Irie, T.; Izumi, H.; Watanabe, T.; Hijikata, A.; Hara, T.; Ohara, O.; et al. Zinc Transporter SLC39A7/ZIP7 Promotes Intestinal Epithelial Self-Renewal by Resolving ER Stress. PLoS Genet. 2016, 12, e1006349. [Google Scholar] [CrossRef] [PubMed]
- Tuncay, E.; Bitirim, V.C.; Durak, A.; Carrat, G.R.J.; Taylor, K.M.; Rutter, G.A.; Turan, B. Hyperglycemia-Induced Changes in ZIP7 and ZnT7 Expression Cause Zn2+ Release from the Sarco(endo)plasmic Reticulum and Mediate ER Stress in the Heart. Diabetes 2017, 66, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Bhin, J.; Seo, J.; Kim, S.Y.; Lee, E.; Park, K.; Choi, D.H.; Takagishi, T.; Hara, T.; Hwang, D.; et al. Requirement of Zinc Transporter SLC39A7/ZIP7 for Dermal Development to Fine-Tune Endoplasmic Reticulum Function by Regulating Protein Disulfide Isomerase. J. Investig. Dermatol. 2017, 137, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Illades, D.; Chen, P.; Zogzas, C.E.; Hutchens, S.; Mercado, J.M.; Swaim, C.D.; Morrisett, R.A.; Bowman, A.B.; Aschner, M.; Mukhopadhyay, S. SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. J. Neurosci. 2014, 34, 14079–14095. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Takeda, T.A.; Nishito, Y. Activation of zinc-requiring ectoenzymes by ZnT transporters during the secretory process: Biochemical and molecular aspects. Arch. Biochem. Biophys. 2016, 611, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ishihara, K.; Migaki, H.; Matsuura, W.; Kohda, A.; Okumura, K.; Nagao, M.; Yamaguchi-Iwai, Y.; Kambe, T. Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J. Biol. Chem. 2005, 280, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kirschke, C.P.; Gitschier, J. Functional characterization of a novel mammalian zinc transporter, ZnT6. J. Biol. Chem. 2002, 277, 26389–26395. [Google Scholar] [CrossRef] [PubMed]
- Kirschke, C.P.; Huang, L. ZnT7, a Novel Mammalian Zinc Transporter, Accumulates Zinc in the Golgi Apparatus. J. Biol. Chem. 2003, 278, 4096–4102. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T. Methods to evaluate zinc transport into and out of the secretory and endosomal-lysosomal compartments in DT40 cells. Methods Enzymol. 2014, 534, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.D.; Macdiarmid, C.W.; Eide, D.J. Heteromeric protein complexes mediate zinc transport into the secretory pathway of eukaryotic cells. J. Biol. Chem. 2005, 280, 28811–28818. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Narita, H.; Yamaguchi-Iwai, Y.; Hirose, J.; Amano, T.; Sugiura, N.; Sasaki, R.; Mori, K.; Iwanaga, T.; Nagao, M. Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J. Biol. Chem. 2002, 277, 19049–19055. [Google Scholar] [CrossRef] [PubMed]
- Kurita, H.; Okuda, R.; Yokoo, K.; Inden, M.; Hozumi, I. Protective roles of SLC30A3 against endoplasmic reticulum stress via ERK1/2 activation. Biochem. Biophys. Res. Commun. 2016, 479, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Go, S.; Kurita, H.; Yokoo, K.; Inden, M.; Kambe, T.; Hozumi, I. Protective function of SLC30A10 induced via PERK-ATF4 pathway against 1-methyl-4-phenylpyridinium. Biochem. Biophys. Res. Commun. 2017. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Nicholson, R.I. Structure-function analysis of HKE4, a member of the new LIV-1 subfamily of zinc transporters. Biochem. J. 2004, 377, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kirschke, C.P.; Zhang, Y.; Yu, Y.Y. The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J. Biol. Chem. 2005, 280, 15456–15463. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Civic, N.; Furuichi, T.; Shimoda, S.; Mishima, K.; Higashiyama, H.; Idaira, Y.; Asada, Y.; Kitamura, H.; Yamasaki, S.; et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS ONE 2008, 3, e3642. [Google Scholar] [CrossRef]
- Matsuura, W.; Yamazaki, T.; Yamaguchi-Iwai, Y.; Masuda, S.; Nagao, M.; Andrews, G.K.; Kambe, T. SLC39A9 (ZIP9) regulates zinc homeostasis in the secretory pathway: Characterization of the ZIP subfamily I protein in vertebrate cells. Biosci. Biotechnol. Biochem. 2009, 73, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pang, Y.; Dong, J.; Berg, A.H. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 2014, 155, 4250–4265. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Walker, J.M.; Wang, F.; Park, J.G.; Palmer, A.E.; Giunta, C.; Rohrbach, M.; Steinmann, B.; Eide, D.J. Promotion of vesicular zinc efflux by ZIP13 and its implications for spondylocheiro dysplastic Ehlers-Danlos syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, E3530–E3538. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.L.; Velasquez, V.; Croxford, T.P.; McCormick, N.H.; Lopez, V.; Macdavid, J. Mapping the zinc-transporting system in mammary cells: Molecular analysis reveals a phenotype-dependent zinc-transporting network during lactation. J. Cell. Physiol. 2012, 227, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Hogstrand, C.; Kille, P.; Nicholson, R.I.; Taylor, K.M. Zinc transporters and cancer: A potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol. Med. 2009, 15, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Hiscox, S.; Nicholson, R.I.; Hogstrand, C.; Kille, P. Protein Kinase CK2 Triggers Cytosolic Zinc Signaling Pathways by Phosphorylation of Zinc Channel ZIP7. Sci. Signal. 2012, 5, ra11. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Fukunaka, A.; Hagihara, M.; Watanabe, K.; Kamino, S.; Kambe, T.; Enomoto, S.; Hiromura, M. Essential Role of the Zinc Transporter ZIP9/SLC39A9 in Regulating the Activations of Akt and Erk in B-Cell Receptor Signaling Pathway in DT40 Cells. PLoS ONE 2013, 8, e58022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumanovics, A.; Poruk, K.E.; Osborn, K.A.; Ward, D.M.; Kaplan, J. YKE4 (YIL023C) encodes a bidirectional zinc transporter in the endoplasmic reticulum of Saccharomyces cerevisiae. J. Biol. Chem. 2006, 281, 22566–22574. [Google Scholar] [CrossRef] [PubMed]
- Stathakis, D.G.; Burton, D.Y.; McIvor, W.E.; Krishnakumar, S.; Wright, T.R.; O’Donnell, J.M. The catecholamines up (Catsup) protein of Drosophila melanogaster functions as a negative regulator of tyrosine hydroxylase activity. Genetics 1999, 153, 361–382. [Google Scholar] [PubMed]
- Groth, C.; Sasamura, T.; Khanna, M.R.; Whitley, M.; Fortini, M.E. Protein trafficking abnormalities in Drosophila tissues with impaired activity of the ZIP7 zinc transporter Catsup. Development 2013, 140, 3018–3027. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Nicholson, R.I. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 2005, 579, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Homma, K.; Fujisawa, T.; Tsuburaya, N.; Yamaguchi, N.; Kadowaki, H.; Takeda, K.; Nishitoh, H.; Matsuzawa, A.; Naguro, I.; Ichijo, H. SOD1 as a Molecular Switch for Initiating the Homeostatic ER Stress Response under Zinc Deficiency. Mol. Cell 2013, 52, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Aydemir, T.B.; Kim, J.; Cousins, R.J. Hepatic ZIP14-mediated zinc transport is required for adaptation to endoplasmic reticulum stress. Proc. Natl. Acad. Sci. USA 2017, 114, E5805–E5814. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Ellis, R.E.; Sakaki, K.; Kaufman, R.J. Genetic Interactions Due to Constitutive and Inducible Gene Regulation Mediated by the Unfolded Protein Response in C. elegans. PLoS Genet. 2005, 1, e37. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M.; Lopez-Otin, C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Reich, R.; Hoffman, A.; Veerendhar, A.; Maresca, A.; Innocenti, A.; Supuran, C.T.; Breuer, E. Carbamoylphosphonates control tumor cell proliferation and dissemination by simultaneously inhibiting carbonic anhydrase IX and matrix metalloproteinase-2. Toward nontoxic chemotherapy targeting tumor microenvironment. J. Med. Chem. 2012, 55, 7875–7882. [Google Scholar] [CrossRef] [PubMed]
- Reich, R.; Hoffman, A.; Suresh, R.R.; Shai, O.; Frant, J.; Maresca, A.; Supuran, C.T.; Breuer, E. Carbamoylphosphonates inhibit autotaxin and metastasis formation in vivo. J. Enzym. Inhib. Med. Chem. 2015, 30, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Itsumura, N.; Kibihara, Y.; Fukue, K.; Miyata, A.; Fukushima, K.; Tamagawa-Mineoka, R.; Katoh, N.; Nishito, Y.; Ishida, R.; Narita, H.; et al. Novel mutations in SLC30A2 involved in the pathogenesis of transient neonatal zinc deficiency. Pediatr. Res. 2016, 80, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Nakagawa, M.; Tsujimura, N.; Miyazaki, S.; Kizu, K.; Goto, T.; Komatsu, Y.; Matsunaga, A.; Shirakawa, H.; Narita, H.; et al. Properties of Zip4 accumulation during zinc deficiency and its usefulness to evaluate zinc status: A study of the effects of zinc deficiency during lactation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R459–R468. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [PubMed]
- Kochanczyk, T.; Drozd, A.; Krezel, A. Relationship between the architecture of zinc coordination and zinc binding affinity in proteins--insights into zinc regulation. Metallomics 2015, 7, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ishihara, K.; Migaki, H.; Nagao, M.; Yamaguchi-Iwai, Y.; Kambe, T. Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J. Biol. Chem. 2005, 280, 30956–30962. [Google Scholar] [CrossRef] [PubMed]
- Lasry, I.; Golan, Y.; Berman, B.; Amram, N.; Glaser, F.; Assaraf, Y.G. In Situ dimerization of multiple wild type and mutant zinc transporters in live cells using bimolecular fluorescence complementation. J. Biol. Chem. 2014, 289, 7275–7292. [Google Scholar] [CrossRef] [PubMed]
- Fukunaka, A.; Kurokawa, Y.; Teranishi, F.; Sekler, I.; Oda, K.; Ackland, M.L.; Faundez, V.; Hiromura, M.; Masuda, S.; Nagao, M.; et al. Tissue Nonspecific Alkaline Phosphatase Is Activated via a Two-step Mechanism by Zinc Transport Complexes in the Early Secretory Pathway. J. Biol. Chem. 2011, 286, 16363–16373. [Google Scholar] [CrossRef] [PubMed]
- McCormick, N.H.; Kelleher, S.L. ZnT4 provides zinc to zinc-dependent proteins in the trans-Golgi network critical for cell function and Zn export in mammary epithelial cells. Am. J. Physiol. Cell Physiol. 2012, 303, C291–C297. [Google Scholar] [CrossRef] [PubMed]
- Murgia, C.; Vespignani, I.; Cerase, J.; Nobili, F.; Perozzi, G. Cloning, expression, and vesicular localization of zinc transporter Dri 27/ZnT4 in intestinal tissue and cells. Am. J. Physiol. 1999, 277, G1231–G1239. [Google Scholar] [PubMed]
- Kukic, I.; Lee, J.K.; Coblentz, J.; Kelleher, S.L.; Kiselyov, K. Zinc-dependent lysosomal enlargement in TRPML1-deficient cells involves MTF-1 transcription factor and ZnT4 (Slc30a4) transporter. Biochem. J. 2013, 451, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gitschier, J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat. Genet. 1997, 17, 292–297. [Google Scholar] [CrossRef] [PubMed]
- McCormick, N.H.; Lee, S.; Hennigar, S.R.; Kelleher, S.L. ZnT4 (SLC30A4)-null (“lethal milk”) mice have defects in mammary gland secretion and hallmarks of precocious involution during lactation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R33–R40. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, S.; Tsuji, T.; Fujiwara, T.; Takeda, T.A.; Merriman, C.; Fukunaka, A.; Nishito, Y.; Fu, D.; Hoch, E.; Sekler, I.; et al. The PP-motif in luminal loop 2 of ZnT transporters plays a pivotal role in TNAP activation. Biochem. J. 2016, 473, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Matsuda, K.; Itoh, M.; Kawaguchi, H.; Tomoike, H.; Aoyagi, T.; Nagai, R.; Hori, M.; Nakamura, Y.; Tanaka, T. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, Znt5. Hum. Mol. Genet. 2002, 11, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Hasegawa, A.; Nakae, S.; Oboki, K.; Saito, H.; Yamasaki, S.; Hirano, T. Zinc transporter Znt5/Slc30a5 is required for the mast cell-mediated delayed-type allergic reaction but not the immediate-type reaction. J. Exp. Med. 2009, 206, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Sheline, C.T.; Shi, C.; Takata, T.; Zhu, J.; Zhang, W.; Sheline, P.J.; Cai, A.L.; Li, L. Dietary zinc reduction, pyruvate supplementation, or zinc transporter 5 knockout attenuates beta-cell death in nonobese diabetic mice, islets, and insulinoma cells. J. Nutr. 2012, 142, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, Y.Y.; Kirschke, C.P.; Gertz, E.R.; Lloyd, K.K. Znt7 (Slc30a7)-deficient mice display reduced body zinc status and body fat accumulation. J. Biol. Chem. 2007, 282, 37053–37063. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kirschke, C.P.; Lay, Y.A.; Levy, L.B.; Lamirande, D.E.; Zhang, P.H. Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance. J. Biol. Chem. 2012, 287, 33883–33896. [Google Scholar] [CrossRef] [PubMed]
- Pound, L.D.; Sarkar, S.A.; Benninger, R.K.; Wang, Y.; Suwanichkul, A.; Shadoan, M.K.; Printz, R.L.; Oeser, J.K.; Lee, C.E.; Piston, D.W.; et al. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem. J. 2009, 421, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, T.J.; Bellomo, E.A.; Wijesekara, N.; Loder, M.K.; Baldwin, J.M.; Gyulkhandanyan, A.V.; Koshkin, V.; Tarasov, A.I.; Carzaniga, R.; Kronenberger, K.; et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 2009, 58, 2070–2083. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, K.; Ravier, M.A.; Schraenen, A.; Creemers, J.W.; Van de Plas, R.; Granvik, M.; Van Lommel, L.; Waelkens, E.; Chimienti, F.; Rutter, G.A.; et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc. Natl. Acad. Sci. USA 2009, 106, 14872–14877. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, N.; Dai, F.F.; Hardy, A.B.; Giglou, P.R.; Bhattacharjee, A.; Koshkin, V.; Chimienti, F.; Gaisano, H.Y.; Rutter, G.A.; Wheeler, M.B. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 2010, 53, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, M.; Fujitani, Y.; Hara, A.; Uchida, T.; Tamura, Y.; Takeno, K.; Kawaguchi, M.; Watanabe, T.; Ogihara, T.; Fukunaka, A.; et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J. Clin. Investig. 2013, 123, 4513–4524. [Google Scholar] [CrossRef] [PubMed]
- Merriman, C.; Huang, Q.; Rutter, G.A.; Fu, D. Lipid-tuned Zinc Transport Activity of Human ZnT8 Protein Correlates with Risk for Type-2 Diabetes. J. Biol. Chem. 2016, 291, 26950–26957. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bai, S.; Sheline, C.T. hZnT8 (Slc30a8) Transgenic Mice That Overexpress the R325W Polymorph Have Reduced Islet Zn2+ and Proinsulin Levels, Increased Glucose Tolerance After a High-Fat Diet, and Altered Levels of Pancreatic Zinc Binding Proteins. Diabetes 2017, 66, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Sladek, R.; Rocheleau, G.; Rung, J.; Dina, C.; Shen, L.; Serre, D.; Boutin, P.; Vincent, D.; Belisle, A.; Hadjadj, S.; et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007, 445, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Flannick, J.; Thorleifsson, G.; Beer, N.L.; Jacobs, S.B.; Grarup, N.; Burtt, N.P.; Mahajan, A.; Fuchsberger, C.; Atzmon, G.; Benediktsson, R.; et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 2014, 46, 357–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabosseau, P.; Rutter, G.A. Zinc and diabetes. Arch Biochem Biophys. Arch. Biochem. Biophys. 2016, 611, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yan, M.; Kirschke, C.P. Over-expression of ZnT7 increases insulin synthesis and secretion in pancreatic beta-cells by promoting insulin gene transcription. Exp. Cell Res. 2010, 316, 2630–2643. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, E.A.; Meur, G.; Rutter, G.A. Glucose regulates free cytosolic Zn2+ concentration, Slc39 (ZiP), and metallothionein gene expression in primary pancreatic islet beta-cells. J. Biol. Chem. 2011, 286, 25778–25789. [Google Scholar] [CrossRef] [PubMed]
- Syring, K.E.; Boortz, K.A.; Oeser, J.K.; Ustione, A.; Platt, K.A.; Shadoan, M.K.; McGuinness, O.P.; Piston, D.W.; Powell, D.R.; O’Brien, R.M. Combined Deletion of Slc30a7 and Slc30a8 Unmasks a Critical Role for ZnT8 in Glucose-Stimulated Insulin Secretion. Endocrinology 2016, 157, 4534–4541. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.B.; Wenzel, H.J.; Kafer, K.E.; Schwartzkroin, P.A.; Palmiter, R.D. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. USA 1999, 96, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Sensi, S.L.; Paoletti, P.; Koh, J.Y.; Aizenman, E.; Bush, A.I.; Hershfinkel, M. The neurophysiology and pathology of brain zinc. J. Neurosci. 2011, 31, 16076–16085. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Nakamura, M.; Fujii, H.; Tamano, H. Synaptic Zn2+ homeostasis and its significance. Metallomics 2013, 5, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Vergnano, A.M.; Rebola, N.; Savtchenko, L.P.; Pinheiro, P.S.; Casado, M.; Kieffer, B.L.; Rusakov, D.A.; Mulle, C.; Paoletti, P. Zinc dynamics and action at excitatory synapses. Neuron 2014, 82, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Hirzel, K.; Muller, U.; Latal, A.T.; Hulsmann, S.; Grudzinska, J.; Seeliger, M.W.; Betz, H.; Laube, B. Hyperekplexia phenotype of glycine receptor alpha1 subunit mutant mice identifies Zn2+ as an essential endogenous modulator of glycinergic neurotransmission. Neuron 2006, 52, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, C.; Vergnano, A.M.; Filliol, D.; Ouagazzal, A.M.; Le Goff, A.; Carvalho, S.; Reiss, D.; Gaveriaux-Ruff, C.; Neyton, J.; Paoletti, P.; et al. Zinc alleviates pain through high-affinity binding to the NMDA receptor NR2A subunit. Nat. Neurosci. 2011, 14, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Parncutt, J.M.; Finkelstein, D.I.; Bush, A.I. Cognitive loss in zinc transporter-3 knock-out mice: A phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J. Neurosci. 2010, 30, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Bush, A.I. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Noguchi, T.; Ikegami, S.; Sakurai, T.; Kakita, A.; Toyoshima, Y.; Kambe, T.; Yamada, M.; Inden, M.; Hara, H.; et al. Zinc transporters ZnT3 and ZnT6 are downregulated in the spinal cords of patients with sporadic amyotrophic lateral sclerosis. J. Neurosci. Res. 2015, 93, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Chowanadisai, W.; Lonnerdal, B.; Kelleher, S.L. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J. Biol. Chem. 2006, 281, 39699–39707. [Google Scholar] [CrossRef] [PubMed]
- Lasry, I.; Seo, Y.A.; Ityel, H.; Shalva, N.; Pode-Shakked, B.; Glaser, F.; Berman, B.; Berezovsky, I.; Goncearenco, A.; Klar, A.; et al. A Dominant Negative Heterozygous G87R Mutation in the Zinc Transporter, ZnT-2 (SLC30A2), Results in Transient Neonatal Zinc Deficiency. J. Biol. Chem. 2012, 287, 29348–29361. [Google Scholar] [CrossRef] [PubMed]
- Itsumura, N.; Inamo, Y.; Okazaki, F.; Teranishi, F.; Narita, H.; Kambe, T.; Kodama, H. Compound Heterozygous Mutations in SLC30A2/ZnT2 Results in Low Milk Zinc Concentrations: A Novel Mechanism for Zinc Deficiency in a Breast-Fed Infant. PLoS ONE 2013, 8, e64045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miletta, M.C.; Bieri, A.; Kernland, K.; Schoni, M.H.; Petkovic, V.; Fluck, C.E.; Eble, A.; Mullis, P.E. Transient Neonatal Zinc Deficiency Caused by a Heterozygous G87R Mutation in the Zinc Transporter ZnT-2 (SLC30A2) Gene in the Mother Highlighting the Importance of Zn2+ for Normal Growth and Development. Int. J. Endocrinol. 2013, 2013, 259189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Michalczyk, A.; McKay, J.; Ford, D.; Kambe, T.; Hudek, L.; Varigios, G.; Taylor, P.E.; Ackland, M.L. Altered expression of two zinc transporters, SLC30A5 and SLC30A6, underlies a mammary gland disorder of reduced zinc secretion into milk. Genes Nutr. 2015, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.M.; Bernhardt, M.L.; Kong, B.Y.; Ahn, R.W.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Biol. 2011, 6, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Que, E.L.; Bleher, R.; Duncan, F.E.; Kong, B.Y.; Gleber, S.C.; Vogt, S.; Chen, S.; Garwin, S.A.; Bayer, A.R.; Dravid, V.P.; et al. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat. Chem. 2015, 7, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Hasegawa, A.; Hojyo, S.; Ohashi, W.; Fukada, T.; Nishida, K.; Hirano, T. A Novel Role of the L-Type Calcium Channel alpha(1D) Subunit as a Gatekeeper for Intracellular Zinc Signaling: Zinc Wave. PLoS ONE 2012, 7, e39654. [Google Scholar] [CrossRef] [PubMed]
- Nimmanon, T.; Ziliotto, S.; Morris, S.; Flanagan, L.; Taylor, K.M. Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling. Metallomics 2017, 9, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Yamasaki, S.; Nishida, K.; Murakami, M.; Hirano, T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J. Biol. Inorg. Chem. 2011, 16, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Chabosseau, P.; Tuncay, E.; Meur, G.; Bellomo, E.A.; Hessels, A.; Hughes, S.; Johnson, P.R.; Bugliani, M.; Marchetti, P.; Turan, B.; et al. Mitochondrial and ER-targeted eCALWY probes reveal high levels of free Zn2+. ACS Chem. Biol. 2014, 9, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Dittmer, P.J.; Park, J.G.; Jansen, K.B.; Palmer, A.E. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc. Natl. Acad. Sci. USA 2011, 108, 7351–7356. [Google Scholar] [CrossRef] [PubMed]




| Enzyme | Active Site | Zn Coordination Residues | ZNTs Involved in Activation | Defects Caused by Loss of ZNTs |
|---|---|---|---|---|
| ALP | Bimetallic center | Asp, His, His for Zn1 His, Asp, Asp for Zn2 | ZNT5-ZNT6, ZNT7 | Loss of enzyme activity Protein destabilization |
| ATX CAIX | Bimetallic center Mononuclear Zn | Asp, His, His for Zn1, His, Asp, Asp for Zn2 His, His, His | ZNT5-ZNT6, ZNT7 ZNT4, ZNT5ZNT6,ZNT7 | Loss of enzyme activity Decreases in enzyme activity |
| MMP-2 MMP-9 | Mononuclear Zn ** | His, His, His | ZNT5-ZNT6, ZNT7 | Loss of enzyme activity Protein destabilization *** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kambe, T.; Matsunaga, M.; Takeda, T.-a. Understanding the Contribution of Zinc Transporters in the Function of the Early Secretory Pathway. Int. J. Mol. Sci. 2017, 18, 2179. https://doi.org/10.3390/ijms18102179
Kambe T, Matsunaga M, Takeda T-a. Understanding the Contribution of Zinc Transporters in the Function of the Early Secretory Pathway. International Journal of Molecular Sciences. 2017; 18(10):2179. https://doi.org/10.3390/ijms18102179
Chicago/Turabian StyleKambe, Taiho, Mayu Matsunaga, and Taka-aki Takeda. 2017. "Understanding the Contribution of Zinc Transporters in the Function of the Early Secretory Pathway" International Journal of Molecular Sciences 18, no. 10: 2179. https://doi.org/10.3390/ijms18102179
APA StyleKambe, T., Matsunaga, M., & Takeda, T. -a. (2017). Understanding the Contribution of Zinc Transporters in the Function of the Early Secretory Pathway. International Journal of Molecular Sciences, 18(10), 2179. https://doi.org/10.3390/ijms18102179