Development of An Impedimetric Aptasensor for the Detection of Staphylococcus aureus
Abstract
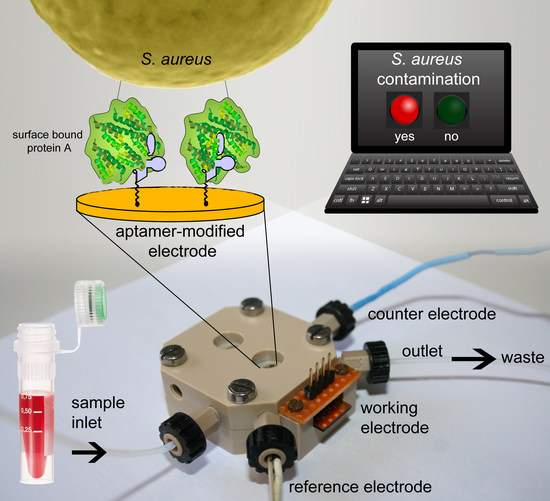
:1. Introduction
2. Results and Discussion
2.1. Functionalization of the Gold Electrodes
2.2. Influence of Ferri-/Ferrocyanide
2.3. Detection of Protein A by Impedance Spectroscopy
2.4. Detection of Staphylococcus aureus by Impedance Spectroscopy
3. Materials and Methods
3.1. Reagents
3.2. Preparation of Electrodes
3.3. QCM Measurements
3.4. MST Measurements
3.5. EIS Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BB | Binding buffer |
| BBT | Binding buffer and 0.005% Tween 20 |
| CFU | Colony-forming unit |
| CPE | Constant phase element |
| DNA | Deoxyribonucleic acid |
| EIS | Electrochemical impedance spectroscopy |
| ELONA | Enzyme-linked oligonucleotide assay |
| FeBB | Binding buffer and 2 mM equimolar ferri-/ferrocyanide |
| h | Hill coefficient |
| KD | Apparent binding constant |
| kDa | kiloDalton |
| LoD | Limit of detection |
| MCH | Mercaptohexanol |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MST | Microscale thermophoresis |
| ON pool | Random oligonucleotide pool |
| PAA | Protein A-binding Aptamer |
| PCR | Polymerase chain reaction |
| QCM | Quartz crystal microbalance |
| Rct/ΔRct | Charge transfer resistance/change of charge transfer resistance |
| RIFTS | Reflective interferometric fourier transform spectroscopy |
| RMS | Rough mean square |
| Rsol | Solution resistance |
| RuHex | hexaammineruthenium(III) chloride |
| SELEX | Systematic Evolution of Ligands by Exponential Enrichment |
| spA | Gene encoding protein A |
| SPR | Surface plasmon resonance spectroscopy |
| SD | Standard deviation |
| TSB | Tryptic soy broth |
| vWF | Von Willebrand factor |
| W | Warburg element |
References
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.C. Rapid diagnostics for methicillin-resistant Staphylococcus aureus: Current status. Mol. Diagn. Ther. 2008, 12, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Yamada, K. Staphylococcal enterotoxins in processed dairy products A2. In Food Hygiene and Toxicology in Ready to Eat Foods; Kotzekidou, P., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 241–258. [Google Scholar]
- Hibnick, H.E.; Bergdoll, M.S. Staphylococcal enterotoxin. II. Chemistry. Arch. Biochem. Biophys. 1959, 85, 70–73. [Google Scholar] [CrossRef]
- DeDent, A.C.; McAdow, M.; Schneewind, O. Distribution of protein A on the surface of Staphylococcus aureus. J. Bacteriol. 2007, 189, 4473–4484. [Google Scholar] [CrossRef] [PubMed]
- Schelin, J.; Wallin-Carlquist, N.; Cohn, M.T.; Lindqvist, R.; Barker, G.C.; Radstrom, P. The formation of Staphylococcus aureus enterotoxin in food environments and advances in risk assessment. Virulence 2011, 2, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Law, J.W.; Ab Mutalib, N.S.; Chan, K.G.; Lee, L.H. Rapid methods for the detection of foodborne bacterial pathogens: Principles, applications, advantages and limitations. Front. Microbiol. 2014, 5, 770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, C.; Zhong, J.; Jin, S. Research advance in rapid detection of foodborne Staphylococcus aureus. Biotechnol. Biotechnol. Equip. 2016, 30, 827–833. [Google Scholar] [CrossRef]
- Oh, A.C.; Lee, J.K.; Lee, H.N.; Hong, Y.J.; Chang, Y.H.; Hong, S.I.; Kim, D.H. Clinical utility of the Xpert MRSA assay for early detection of methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2013, 7, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.P.; Kougianos, E. Biosensors: A tutorial review. IEEE Potentials 2006, 25, 35–40. [Google Scholar] [CrossRef]
- Hoja, J.; Lentka, G. A family of new generation miniaturized impedance analyzers for technical object diagnostics. Metrol. Meas. Syst. 2013, 20. [Google Scholar] [CrossRef]
- Lisdat, F.; Schafer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Bahadir, E.B.; Sezginturk, M.K. A review on impedimetric biosensors. Artif. Cells Nanomed. Biotechnol. 2016, 44, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Bekir, K.; Barhoumi, H.; Braiek, M.; Chrouda, A.; Zine, N.; Abid, N.; Maaref, A.; Bakhrouf, A.; Ouada, H.B.; Jaffrezic-Renault, N.; et al. Electrochemical impedance immunosensor for rapid detection of stressed pathogenic Staphylococcus aureus bacteria. Environ. Sci. Pollut. Res. Int. 2015, 22, 15796–15803. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.K. Aptasensors—The future of biosensing? Anal. Bioanal. Chem. 2002, 372, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.C. Methods developed for SELEX. Anal. Bioanal. Chem. 2007, 387, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Zayats, M. Electronic aptamer-based sensors. Angew. Chem. Int. Ed. Engl. 2007, 46, 6408–6418. [Google Scholar] [CrossRef] [PubMed]
- Radi, A.E.; Acero Sanchez, J.L.; Baldrich, E.; O’Sullivan, C.K. Reusable impedimetric aptasensor. Anal. Chem. 2005, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Booth, M.A.; Woo, H.Y.; Hodgkiss, J.M.; Travas-Sejdic, J. Label-Free, electrochemical quantitation of potassium ions from femtomolar levels. Chem. Asian. J. 2015, 10, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Man, Y.; Jin, X.; Pan, L.; Liu, X. Aptamer-based biosensor for label-free detection of ethanolamine by electrochemical impedance spectroscopy. Anal. Chim. Acta. 2016, 936, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Zamay, A.S.; Kolovskaya, O.S.; Reshetneva, I.T.; Zamay, G.S.; Kibbee, R.J.; Sattar, S.A.; Zamay, T.N.; Berezovski, M.V. Aptamer-based viability impedimetric sensor for bacteria. Anal. Chem. 2012, 84, 8966–8969. [Google Scholar] [CrossRef] [PubMed]
- Shahdordizadeh, M.; Taghdisi, S.M.; Ansari, N.; Langroodi, F.A.; Abnous, K.; Ramezani, M. Aptamer based biosensors for detection of Staphylococcus aureus. Sens. Actuators B Chem. 2017, 241, 619–635. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, S.; Duan, N.; Ma, X.; Xia, Y.; Chen, J.; Ding, Z.; Wang, Z. A sensitive gold nanoparticle-based colorimetric aptasensor for Staphylococcus aureus. Talanta 2014, 127, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, J.; Li, Y.; He, D.; He, X.; Wang, K.; Zou, Z.; Shi, H. A combination of positive dielectrophoresis driven on-line enrichment and aptamer-fluorescent silica nanoparticle label for rapid and sensitive detection of Staphylococcus aureus. Analyst 2015, 140, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Wu, S.; Zhu, C.; Ma, X.; Wang, Z.; Yu, Y.; Jiang, Y. Dual-color upconversion fluorescence and aptamer-functionalized magnetic nanoparticles-based bioassay for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Anal. Chim. Acta 2012, 723, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Yang, C.Y.; Sun, R.L.; Cheng, Y.F.; Kao, W.C.; Yang, P.C. Rapid single cell detection of Staphylococcus aureus by aptamer-conjugated gold nanoparticles. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Zelada-Guillen, G.A.; Sebastian-Avila, J.L.; Blondeau, P.; Riu, J.; Rius, F.X. Label-free detection of Staphylococcus aureus in skin using real-time potentiometric biosensors based on carbon nanotubes and aptamers. Biosens. Bioelectron. 2012, 31, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Valles, C.; Benito, A.M.; Maser, W.K.; Rius, F.X.; Riu, J. Graphene-based potentiometric biosensor for the immediate detection of living bacteria. Biosens. Bioelectron. 2014, 54, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, A.; Norouz-Sarvestani, F.; Noori, A.; Soltani, N. Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of Staphylococcus aureus. Biosens. Bioelectron. 2015, 68. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; He, F.; Wang, H.; Tong, F. A new aptamer/graphene interdigitated gold electrode piezoelectric sensor for rapid and specific detection of Staphylococcus aureus. Biosens. Bioelectron. 2015, 65, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Duan, N.; Wu, S.; Ma, X.; Xia, Y.; Wang, Z.; Wei, X. Impedimetric aptasensor for Staphylococcus aureus based on nanocomposite prepared from reduced graphene oxide and gold nanoparticles. Microchim. Acta 2014, 181, 967–974. [Google Scholar] [CrossRef]
- Kahl, B.C.; Mellmann, A.; Deiwick, S.; Peters, G.; Harmsen, D. Variation of the polymorphic region X of the protein A gene during persistent airway infection of cystic fibrosis patients reflects two independent mechanisms of genetic change in Staphylococcus aureus. J. Clin. Microbiol. 2005, 43, 502–505. [Google Scholar] [CrossRef] [PubMed]
- O’Seaghdha, M.; van Schooten, C.J.; Kerrigan, S.W.; Emsley, J.; Silverman, G.J.; Cox, D.; Lenting, P.J.; Foster, T.J. Staphylococcus aureus protein A binding to von Willebrand factor A1 domain is mediated by conserved IgG binding regions. FEBS J. 2006, 273, 4831–4841. [Google Scholar] [CrossRef] [PubMed]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. FluMag-SELEX as an advantageous method for DNA aptamer selection. Anal. Bioanal. Chem. 2005, 383, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Stoltenburg, R.; Schubert, T.; Strehlitz, B. In vitro selection and interaction studies of a DNA aptamer targeting protein A. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Stoltenburg, R.; Krafcikova, P.; Viglasky, V.; Strehlitz, B. G-quadruplex aptamer targeting protein A and its capability to detect Staphylococcus aureus demonstrated by ELONA. Sci. Rep. 2016, 6, 33812. [Google Scholar] [CrossRef] [PubMed]
- Keighley, S.D.; Li, P.; Estrela, P.; Migliorato, P. Optimization of DNA immobilization on gold electrodes for label-free detection by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2008, 23, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Steel, A.B.; Herne, T.M.; Tarlov, M.J. Electrochemical quantitation of DNA immobilized on gold. Anal. Chem. 1998, 70, 4670–4677. [Google Scholar] [CrossRef] [PubMed]
- Debono, R.F.; Loucks, G.D.; Manna, D.D.; Krull, U.J. Self-assembly of short and long-chain n-alkyl thiols onto gold surfaces: A real-time study using surface plasmon resonance techniques. Can. J. Chem. 1996, 74, 677–688. [Google Scholar] [CrossRef]
- Martin, S.J.; Granstaff, V.E.; Frye, G.C. Characterization of a quartz crystal microbalance with simultaneous mass and liquid loading. Anal. Chem. 2002, 63, 2272–2281. [Google Scholar] [CrossRef]
- Urmann, K.; Modrejewski, J.; Scheper, T.; Walter Johanna, G. Aptamer-modified nanomaterials: Principles and applications. BioNanoMaterials 2017, 18. [Google Scholar] [CrossRef]
- Erickson, H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Angell, D.H.; Dickinson, T. The kinetics of the ferrous/ferric and ferro/ferricyanide reactions at platinum and gold electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1972, 35, 55–72. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Sjödahl, J. Structural studies on the four repetitive Fc-binding regions in protein A from Staphylococcus aureus. Eur. J. Biochem. 1977, 78, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Urmann, K.; Reich, P.; Walter, J.G.; Beckmann, D.; Segal, E.; Scheper, T. Rapid and label-free detection of protein A by aptamer-tethered porous silicon nanostructures. J. Biotechnol. 2017, 257, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Banada, P.P.; Chakravorty, S.; Shah, D.; Burday, M.; Mazzella, F.M.; Alland, D. Highly sensitive detection of Staphylococcus aureus directly from patient blood. PLoS ONE 2012, 7, e31126. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, S.; Chen, L.; Ding, H.; Xu, H.; Huang, Y.; Li, J.; Liu, N.; Cao, W.; Zhu, Y.; et al. Combining use of a panel of ssDNA aptamers in the detection of Staphylococcus aureus. Nucleic Acids Res. 2009, 37, 4621–4628. [Google Scholar] [CrossRef] [PubMed]
- Reich, P. Entwicklung eines impedimetrischen Aptasensor zur Detektion von Staphylococcus aureus. Ph.D. Thesis, Leibniz Universität Hannover, Hannover, Germany, 2017. in preparation. [Google Scholar]
- Tallent, S.M.; Bennett, R.W.; Hait, J.M. Chapter 13 B Staphylococcal Enterotoxins Detection Methods. 2017; U.S. Food and Drug Administration. Available online: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm564359.htm (accessed on 13 November 2017).
- Bennett, R.W.; Lancette, G.A. Chapter 12 Staphylococcus aureus. 2016; U.S. Food and Drug Administration. Available online: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm071429.htm (accessed on 13 November 2017).
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Zeitschrift für Physik 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Oesch, U.; Janata, J. Electrochemical study of gold electrodes with anodic oxide films—I. Formation and reduction behaviour of anodic oxides on gold. Electrochim. Acta 1983, 28, 1237–1246. [Google Scholar] [CrossRef]







| c(Protein A)/nM | Rsol/Ω·cm2 | SD | CPE/µF·s(α-1) | SD | α | Rct/Ω·cm2 | SD | σ/Ω·s−1/2 | SD | Χ/ |
|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 44.4 | 0.2 | 0.362 | 0.005 | 0.95 | 505.3 | 0.5 | 5769 | 2 | 0.0122 |
| 24 | 46.2 | 0.1 | 0.375 | 0.004 | 0.94 | 593.3 | 0.5 | 5847 | 2 | 0.0124 |
| 70 | 45.4 | 0.1 | 0.359 | 0.004 | 0.95 | 627.9 | 0.5 | 5861 | 2 | 0.0120 |
| 240 | 46.1 | 0.1 | 0.341 | 0.004 | 0.95 | 647.3 | 0.5 | 5834 | 2 | 0.0105 |
| 700 | 45.3 | 0.1 | 0.361 | 0.004 | 0.95 | 657.2 | 0.5 | 5811 | 2 | 0.0118 |
| Analysis | Aptamer | Aptamer | Protein A | KD/nM | Reference | ||
|---|---|---|---|---|---|---|---|
| Method | Modification | ||||||
| MST | free | 5′-fluorescence | free | 94.7 | ± | 64.6 | [35] |
| MST | free | 5′-fluorescence | free | 115.6 | ± | 26.9 | this work |
| MST | free | 3′-fluorescence | free | 110.8 | ± | 42.3 | this work |
| MST | free | 3′-fluorescence | free | 22.4 | ± | 5.8 | this work |
| SPR | free | 5′-fluorescence | immobilized | 1920.0 | ± | 250.0 | [35] |
| SPR | immobilized | 3′-biotin | free | 287.0 | ± | 16.2 | [35] |
| ELONA | free | 5′-biotin | immobilized | 23.7 | ± | 2.0 | [36] |
| ELONA | free | 3′-biotin | immobilized | 11.3 | ± | 1.4 | [36] |
| EIS | immobilized | 3′-thiol | free | 18.5 | ± | 1.8 | this work |
| RIFTS | immobilized | 3′-amino | free | 13980.0 | ± | 1540.0 | [46] |
| c(S. aureus) /CFU·mL−1 | Rsol /Ω·cm2 | SD | CPE /µF·s(α-1) | SD | α | Rct /Ω·cm2 | SD | σ /Ω·s−1/2 | SD | Χ/ |
|---|---|---|---|---|---|---|---|---|---|---|
| 1E+01 | 14.8 | 0.1 | 1.230 | 0.02 | 0.99 | 459.8 | 0.3 | 310 | 3 | 0.0084 |
| 1E+02 | 14.8 | 0.1 | 1.232 | 0.02 | 0.99 | 467.8 | 0.3 | 311 | 3 | 0.0092 |
| 1E+03 | 14.9 | 0.1 | 1.229 | 0.02 | 0.99 | 474.7 | 0.3 | 312 | 3 | 0.0088 |
| 1E+04 | 15.0 | 0.1 | 1.224 | 0.02 | 0.99 | 485.7 | 0.3 | 315 | 3 | 0.0086 |
| 1E+05 | 15.0 | 0.1 | 1.232 | 0.02 | 0.99 | 492.2 | 0.3 | 312 | 3 | 0.0098 |
| 1E+08 | 14.9 | 0.1 | 1.231 | 0.02 | 0.99 | 497.9 | 0.3 | 312 | 3 | 0.0098 |
| 1E+10 | 14.9 | 0.1 | 1.226 | 0.02 | 0.99 | 491.8 | 0.3 | 317 | 3 | 0.0094 |
| Detection Principle | Recognition Element | Assay Time | LoD/CFU·mL−1 | Reference |
|---|---|---|---|---|
| polymerase chain reaction | ssDNA | 2 h | 10 | [47] |
| EIS immunosensor | anti-S. aureus-antibody | Not stated | 10 | [14] |
| resonance light scattering | SA 17 & SA 61 [26] | 1.5 h | 1 | [26] |
| EIS | SA 43 [48] | 1 h | 10 | [31] |
| fluorescent nanoparticles | SA 31 [48] | 2 h | 93 | [24] |
| EIS | PA2#8[S1–58] [35] | 10 min | 10 | this work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reich, P.; Stoltenburg, R.; Strehlitz, B.; Frense, D.; Beckmann, D. Development of An Impedimetric Aptasensor for the Detection of Staphylococcus aureus. Int. J. Mol. Sci. 2017, 18, 2484. https://doi.org/10.3390/ijms18112484
Reich P, Stoltenburg R, Strehlitz B, Frense D, Beckmann D. Development of An Impedimetric Aptasensor for the Detection of Staphylococcus aureus. International Journal of Molecular Sciences. 2017; 18(11):2484. https://doi.org/10.3390/ijms18112484
Chicago/Turabian StyleReich, Peggy, Regina Stoltenburg, Beate Strehlitz, Dieter Frense, and Dieter Beckmann. 2017. "Development of An Impedimetric Aptasensor for the Detection of Staphylococcus aureus" International Journal of Molecular Sciences 18, no. 11: 2484. https://doi.org/10.3390/ijms18112484
APA StyleReich, P., Stoltenburg, R., Strehlitz, B., Frense, D., & Beckmann, D. (2017). Development of An Impedimetric Aptasensor for the Detection of Staphylococcus aureus. International Journal of Molecular Sciences, 18(11), 2484. https://doi.org/10.3390/ijms18112484