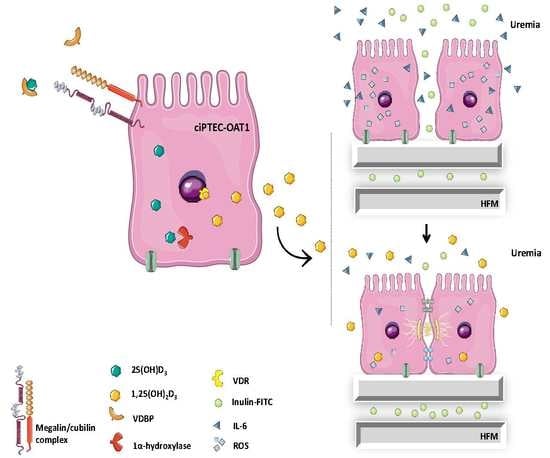
Role of Vitamin D in Maintaining Renal Epithelial Barrier Function in Uremic Conditions
Abstract
:1. Introduction
2. Results
2.1. Expression of Vitamin D Metabolism and Function-Related Genes in ciPTEC-OAT1
2.2. Conversion of 25(OH)D3 to 1,25(OH)2D3 by ciPTEC-OAT1
2.3. Protective Effect of 1,25(OH)2D3 on Anionic Uremic Toxin Mix Induced Cell Toxicity
2.4. Protective Effect of 1,25(OH)2D3 on Anionic Uremic Toxin Mix Induced Oxidative Stress
2.5. Anti-Inflammatory Effect of 1,25(OH)2D3 in Inflammatory and Uremic Conditions in ciPTEC-OAT1
2.6. Beneficial Effect of 1,25(OH)2D3 on ciPTEC-OAT1 Epithelial Barrier Formation on HFM
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture of ciPTEC-OAT1
4.3. ciPTEC-OAT1 Exposure to Uremic Toxins Mixture
4.4. Cell Viability Assay
4.5. RNA Extraction, cDNA Synthesis, and Real-Time PCR
4.6. Agarose Gel Electrophoresis
4.7. Quantification of 1α,25-Dihydroxy-Vitamin D3
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Intracellular Reactive Oxygen Species (ROS) Detection
4.10. CiPTEC-OAT1 Epithelial Monolayer Integrity
4.11. Immunocytochemistry
4.12. Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Covic, A.; Fliser, D.; Fouque, D.; Goldsmith, D.; Kanbay, M.; Mallamaci, F.; Massy, Z.A.; Rossignol, P.; Vanholder, R.; et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014, 383, 1831–1843. [Google Scholar] [CrossRef]
- Vanholder, R.; Baurmeister, U.; Brunet, P.; Cohen, G.; Glorieux, G.; Jankowski, J. A bench to bedside view of uremic toxins. J. Am. Soc. Nephrol. 2008, 19, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Deltombe, O.; Van Biesen, W.; Glorieux, G.; Massy, Z.; Dhondt, A.; Eloot, S. Exploring Protein Binding of Uremic Toxins in Patients with Different Stages of Chronic Kidney Disease and during Hemodialysis. Toxins 2015, 7, 3933–3946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalier, E.; Torres, P.U.; Dubois, B.E.; Smelten, N.; Pottel, H.; Krzesinski, J.M.; Delanaye, P. Impact of the type of dialysis membranes on the circulating concentration of markers of vitamin D metabolism. Int. J. Artif. Organs 2017, 40, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Kanso, A.; Sedor, J.R. Chronic kidney disease and its complications. Prim. Care 2008, 35, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S. Kidney disease and vitamin D levels: 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and VDR activation. Kidney Int. Suppl. 2011, 1, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Tokumoto, M. Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: A downward spiral in kidney disease. Kidney Int. 2011, 79, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Artaza, J.N.; Mehrotra, R.; Norris, K.C. Vitamin D and the cardiovascular system. Clin. J. Am. Soc. Nephrol. 2009, 4, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Gouni-Berthold, I.; Krone, W.; Berthold, H.K. Vitamin D and cardiovascular disease. Curr. Vasc. Pharmacol. 2009, 7, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Schuster, I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim. Biophys. Acta 2011, 1814, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-Hydroxyvitamin d-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Al-Badr, W.; Martin, K.J. Vitamin D and kidney disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.J.; Zhou, T.B.; Zhang, Y.F.; Wang, Q.; Su, Y.Y.; Tang, J.M.; Li, H.Y. Levels of vitamin D receptor and CYP24A1 in patients with end-stage renal disease. Afr. Health Sci. 2016, 16, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Melamed, M.L.; Astor, B.; Michos, E.D.; Hostetter, T.H.; Powe, N.R.; Muntner, P. 25-hydroxyvitamin D levels, race, and the progression of kidney disease. J. Am. Soc. Nephrol. 2009, 20, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Assa, A.; Vong, L.; Pinnell, L.J.; Avitzur, N.; Johnson-Henry, K.C.; Sherman, P.M. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J. Infect. Dis. 2014, 210, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Fedecostante, M.; Wilmer, M.J.; van den Heuvel, L.P.; Hoenderop, J.G.; Masereeuw, R. Biotechnological challenges of bioartificial kidney engineering. Biotechnol. Adv. 2014, 32, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Humes, H.D.; Buffington, D.A.; MacKay, S.M.; Funke, A.J.; Weitzel, W.F. Replacement of renal function in uremic animals with a tissue-engineered kidney. Nat. Biotechnol. 1999, 17, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Humes, H.D.; MacKay, S.M.; Funke, A.J.; Buffington, D.A. Tissue engineering of a bioartificial renal tubule assist device: In vitro transport and metabolic characteristics. Kidney Int. 1999, 55, 2502–2514. [Google Scholar] [CrossRef] [PubMed]
- Fissell, W.H.; Lou, L.; Abrishami, S.; Buffington, D.A.; Humes, H.D. Bioartificial kidney ameliorates gram-negative bacteria-induced septic shock in uremic animals. J. Am. Soc. Nephrol. 2003, 14, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Kakuta, T.; Asano, M.; Itoh, J.; Sakabe, K.; Tokimasa, T.; Saito, A. Evaluation of Na+ active transport and morphological changes for bioartificial renal tubule cell device using Madin-Darby canine kidney cells. Tissue Eng. 2002, 8, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Terashima, M.; Kakuta, T.; Itoh, J.; Tokimasa, T.; Brown, D.; Saito, A. Transcellular water transport and stability of expression in aquaporin 1-transfected LLC-PK1 cells in the development of a portable bioartificial renal tubule device. Tissue Eng. 2004, 10, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Humes, H.D.; Weitzel, W.F.; Bartlett, R.H.; Swaniker, F.C.; Paganini, E.P.; Luderer, J.R.; Sobota, J. Initial clinical results of the bioartificial kidney containing human cells in ICU patients with acute renal failure. Kidney Int. 2004, 66, 1578–1588. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, M.J.; Saleem, M.A.; Masereeuw, R.; Ni, L.; van der Velden, T.J.; Russel, F.G.; Mathieson, P.W.; Monnens, L.A.; van den Heuvel, L.P.; Levtchenko, E.N. Novel conditionally immortalized human proximal tubule cell line expressing functional influx and efflux transporters. Cell Tissue Res. 2010, 339, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.; Schophuizen, C.M.; Wilmer, M.J.; Lahham, S.H.; Mutsaers, H.A.; Wetzels, J.F.; Bank, R.A.; van den Heuvel, L.P.; Hoenderop, J.G.; Masereeuw, R. A morphological and functional comparison of proximal tubule cell lines established from human urine and kidney tissue. Exp. Cell Res. 2014, 323, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Nieskens, T.T.; Peters, J.G.; Schreurs, M.J.; Smits, N.; Woestenenk, R.; Jansen, K.; van der Made, T.K.; Roring, M.; Hilgendorf, C.; Wilmer, M.J.; et al. A Human Renal Proximal Tubule Cell Line with Stable Organic Anion Transporter 1 and 3 Expression Predictive for Antiviral-Induced Toxicity. AAPS J. 2016, 18, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; de Napoli, I.E.; Fedecostante, M.; Schophuizen, C.M.; Chevtchik, N.V.; Wilmer, M.J.; van Asbeck, A.H.; Croes, H.J.; Pertijs, J.C.; Wetzels, J.F.; et al. Human proximal tubule epithelial cells cultured on hollow fibers: Living membranes that actively transport organic cations. Sci. Rep. 2015, 5, 16702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schophuizen, C.M.; de Napoli, I.E.; Jansen, J.; Teixeira, S.; Wilmer, M.J.; Hoenderop, J.G.; van den Heuvel, L.P.; Masereeuw, R.; Stamatialis, D. Development of a living membrane comprising a functional human renal proximal tubule cell monolayer on polyethersulfone polymeric membrane. Acta Biomater. 2015, 14, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Fedecostante, M.; Wilmer, M.J.; Peters, J.G.; Kreuser, U.M.; van den Broek, P.H.; Mensink, R.A.; Boltje, T.J.; Stamatialis, D.; Wetzels, J.F.; et al. Bioengineered kidney tubules efficiently excrete uremic toxins. Sci. Rep. 2016, 6, 26715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.; Jankowski, J.; Gajjala, P.R.; Wetzels, J.F.M.; Masereeuw, R. Disposition and clinical implications of protein-bound uremic toxins. Clin. Sci. 2017, 131, 1631–1647. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, D.; Bland, R.; Walker, E.A.; Bradwell, A.R.; Howie, A.J.; Hewison, M.; Stewart, P.M. Expression of 25-hydroxyvitamin D3–1alpha-hydroxylase in the human kidney. J. Am. Soc. Nephrol. 1999, 10, 2465–2473. [Google Scholar] [PubMed]
- Bikle, D.D.; Nemanic, M.K.; Gee, E.; Elias, P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J. Clin. Investig. 1986, 78, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Tangpricha, V.; Flanagan, J.N.; Whitlatch, L.W.; Tseng, C.C.; Chen, T.C.; Holt, P.R.; Lipkin, M.S.; Holick, M.F. 25-hydroxyvitamin D-1alpha-hydroxylase in normal and malignant colon tissue. Lancet 2001, 357, 1673–1674. [Google Scholar] [CrossRef]
- Kemmis, C.M.; Salvador, S.M.; Smith, K.M.; Welsh, J. Human mammary epithelial cells express CYP27B1 and are growth inhibited by 25-hydroxyvitamin D-3, the major circulating form of vitamin D-3. J. Nutr. 2006, 136, 887–892. [Google Scholar] [PubMed]
- Adams, J.S.; Sharma, O.P.; Gacad, M.A.; Singer, F.R. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J. Clin. Investig. 1983, 72, 1856–1860. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, M.; Andreesen, R.; Krause, S.W.; Szabo, A.; Ritz, E.; Reichel, H. 1,25-dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood 1993, 82, 1300–1307. [Google Scholar] [PubMed]
- Sigmundsdottir, H.; Pan, J.; Debes, G.F.; Alt, C.; Habtezion, A.; Soler, D.; Butcher, E.C. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 2007, 8, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Hansdottir, S.; Monick, M.M.; Hinde, S.L.; Lovan, N.; Look, D.C.; Hunninghake, G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: Potential effects on host defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar] [CrossRef] [PubMed]
- Murayama, A.; Takeyama, K.; Kitanaka, S.; Kodera, Y.; Hosoya, T.; Kato, S. The promoter of the human 25-hydroxyvitamin D3 1 alpha-hydroxylase gene confers positive and negative responsiveness to PTH, calcitonin, and 1 alpha,25(OH)2D3. Biochem. Biophys. Res. Commun. 1998, 249, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Yoshimura, T.; Iemura, O.; Yamada, E.; Tsujikawa, K.; Kohama, Y.; Mimura, T. Molecular cloning of 25-hydroxyvitamin D-3 24-hydroxylase (Cyp-24) from mouse kidney: Its inducibility by vitamin D-3. Biochim. Biophys. Acta 1995, 1264, 26–28. [Google Scholar] [CrossRef]
- Bikle, D.D.; Gee, E.; Halloran, B.; Haddad, J.G. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J. Clin. Investig. 1984, 74, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Biancuzzo, R.M.; Clarke, N.; Reitz, R.E.; Travison, T.G.; Holick, M.F. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J. Clin. Endocrinol. Metab. 2013, 98, 973–979. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [PubMed]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, N.M. The noncalciotropic actions of vitamin D: Recent clinical developments. Curr. Opin. Nephrol. Hypertens. 2008, 17, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Nursyam, E.W.; Amin, Z.; Rumende, C.M. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med. Indones. 2006, 38, 3–5. [Google Scholar] [PubMed]
- Zasloff, M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J. Am. Soc. Nephrol. 2007, 18, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernan, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.S. Vitamin D and type 1 diabetes. Am. J. Clin. Nutr. 2004, 79, 889–890. [Google Scholar] [PubMed]
- Alarcon, G.S.; Friedman, A.W.; Straaton, K.V.; Moulds, J.M.; Lisse, J.; Bastian, H.M.; McGwin, G., Jr.; Bartolucci, A.A.; Roseman, J.M.; Reveille, J.D. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs. Nurture. Lupus 1999, 8, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Hayes, C.E.; DeLuca, H.F. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J. Nutr. 1998, 128, 68–72. [Google Scholar] [PubMed]
- Samuel, S.; Sitrin, M.D. Vitamin D’s role in cell proliferation and differentiation. Nutr. Rev. 2008, 66, S116–S124. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Takeda, M.; Tojo, A.; Sekine, T.; Cha, S.H.; Khamdang, S.; Takayama, F.; Aoyama, I.; Nakamura, S.; Endou, H.; et al. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J. Am. Soc. Nephrol. 2002, 13, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Stockler-Pinto, M.B.; Saldanha, J.F.; Yi, D.; Mafra, D.; Fouque, D.; Soulage, C.O. The uremic toxin indoxyl sulfate exacerbates reactive oxygen species production and inflammation in 3T3-L1 adipose cells. Free Radic. Res. 2016, 50, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, I.; Tatebe, J.; Namba, S.; Koizumi, M.; Yamazaki, J.; Morita, T. Activation of aryl hydrocarbon receptor mediates indoxyl sulfate-induced monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells. Circ. J. 2013, 77, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Sallee, M.; Cerini, C.; Poitevin, S.; Gondouin, B.; Jourde-Chiche, N.; Fallague, K.; Brunet, P.; Calaf, R.; Dussol, B.; et al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J. Am. Soc. Nephrol. 2015, 26, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Campbell, K.L.; Johnson, D.W.; Stanton, T.; Vesey, D.A.; Coombes, J.S.; Weston, K.S.; Hawley, C.M.; McWhinney, B.C.; Ungerer, J.P.; et al. Protein-bound uremic toxins, inflammation and oxidative stress: A cross-sectional study in stage 3–4 chronic kidney disease. Arch. Med. Res. 2014, 45, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Tumur, Z.; Shimizu, H.; Enomoto, A.; Miyazaki, H.; Niwa, T. Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kappaB activation. Am. J. Nephrol. 2010, 31, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Osaka, M.; Higuchi, Y.; Nishijima, F.; Ishii, H.; Yoshida, M. Indoxyl sulfate induces leukocyte-endothelial interactions through up-regulation of E-selectin. J. Biol. Chem. 2010, 285, 38869–38875. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Jourde-Chiche, N.; Faure, V.; Cerini, C.; Berland, Y.; Dignat-George, F.; Brunet, P. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J. Thromb. Haemost. 2007, 5, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Bolati, D.; Shimizu, H.; Yisireyili, M.; Nishijima, F.; Niwa, T. Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-kappaB. BMC Nephrol. 2013, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Borges, N.A.; Barros, A.F.; Nakao, L.S.; Dolenga, C.J.; Fouque, D.; Mafra, D. Protein-Bound Uremic Toxins from Gut Microbiota and Inflammatory Markers in Chronic Kidney Disease. J. Ren. Nutr. 2016, 26, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Finch, J.L.; Suarez, E.B.; Husain, K.; Ferder, L.; Cardema, M.C.; Glenn, D.J.; Gardner, D.G.; Liapis, H.; Slatopolsky, E. Effect of combining an ACE inhibitor and a VDR activator on glomerulosclerosis, proteinuria, and renal oxidative stress in uremic rats. Am. J. Phys. Ren. Phys. 2012, 302, F141–F149. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.J.; Cavia, M.; Muniz, P.; de Francisco, A.L.; Arias, M.; Santos, J.; Abaigar, P. Paricalcitol reduces oxidative stress and inflammation in hemodialysis patients. BMC Nephrol. 2012, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Gonzalez, J.F.; Donate-Correa, J.; Mendez, M.L.; de Fuentes, M.M.; Garcia-Perez, J.; Mora-Fernandez, C. Anti-inflammatory profile of paricalcitol in hemodialysis patients: A prospective, open-label, pilot study. J. Clin. Pharmacol. 2013, 53, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, Y.; Golan, M.A.; Annunziata, M.L.; Du, J.; Dougherty, U.; Kong, J.; Musch, M.; Huang, Y.; Pekow, J.; et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J. Clin. Investig. 2013, 123, 3983–3996. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Pintea, V.; Lin, Y.; Hammock, B.D.; Watsky, M.A. Vitamin D enhances corneal epithelial barrier function. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7359–7364. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Y.; Liu, T.J.; Fu, J.H.; Xu, W.; Wu, L.L.; Hou, A.N.; Xue, X.D. Vitamin D/VDR signaling attenuates lipopolysaccharideinduced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol. Med. Rep. 2016, 13, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.S. Claudins and the kidney. J. Am. Soc. Nephrol. 2015, 26, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kirk, A.; Campbell, S.; Bass, P.; Mason, J.; Collins, J. Differential expression of claudin tight junction proteins in the human cortical nephron. Nephrol. Dial. Transplant. 2010, 25, 2107–2119. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, M.; van den Heuvel, L.P.; Hoenderop, J.G.; Jansen, J.; Wilmer, M.J.; Westheim, A.J.F.; Allebes, W.A.; Stamatialis, D.; Hilbrands, L.B.; Masereeuw, R. Allostimulatory capacity of conditionally immortalized proximal tubule cell lines for bioartificial kidney application. Sci. Rep. 2017, 7, 7103. [Google Scholar] [CrossRef] [PubMed]
- Chevtchik, N.V.; Fedecostante, M.; Jansen, J.; Mihajlovic, M.; Wilmer, M.; Ruth, M.; Masereeuw, R.; Stamatialis, D. Upscaling of a living membrane for bioartificial kidney device. Eur. J. Pharmacol. 2016, 790, 28–35. [Google Scholar] [CrossRef] [PubMed]






| Compound | Normal conc. (μM) (Mean ± SD) | Uremic conc. (μM) (Mean ± SD) | 1× UT mix (μM) | Structure |
|---|---|---|---|---|
| Indoxyl sulfate | 2.3 ± 18.8 | 173.5 ± 121.9 | 100 |  |
| p-cresyl sulfate | 10.1 ± 12.2 | 122.2 ± 90.3 | 500 |  |
| Indoxyl-β-glucuronide | 3.1 ± 1.3 | 9.4 ± 9.4 | 10 |  |
| p-cresyl glucuronide | 0.3 ± 0.2 | 30.1 ± 6.7 | 40 |  |
| Indol-3-acetic acid | 2.9 ± 1.7 | 11.4 ± 2.3 | 3 |  |
| Hippuric acid | 16.7 ± 11.2 | 608.4 ± 362.8 | 300 |  |
| Kynurenic acid | 0.03 ± 0.01 | 0.8 ± 0.4 | 3 |  |
| L-kynurenine | 1.9 | 3.3 ± 0.9 | 5 |  |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihajlovic, M.; Fedecostante, M.; Oost, M.J.; Steenhuis, S.K.P.; Lentjes, E.G.W.M.; Maitimu-Smeele, I.; Janssen, M.J.; Hilbrands, L.B.; Masereeuw, R. Role of Vitamin D in Maintaining Renal Epithelial Barrier Function in Uremic Conditions. Int. J. Mol. Sci. 2017, 18, 2531. https://doi.org/10.3390/ijms18122531
Mihajlovic M, Fedecostante M, Oost MJ, Steenhuis SKP, Lentjes EGWM, Maitimu-Smeele I, Janssen MJ, Hilbrands LB, Masereeuw R. Role of Vitamin D in Maintaining Renal Epithelial Barrier Function in Uremic Conditions. International Journal of Molecular Sciences. 2017; 18(12):2531. https://doi.org/10.3390/ijms18122531
Chicago/Turabian StyleMihajlovic, Milos, Michele Fedecostante, Miriam J. Oost, Sonja K. P. Steenhuis, Eef G. W. M. Lentjes, Inge Maitimu-Smeele, Manoe J. Janssen, Luuk B. Hilbrands, and Rosalinde Masereeuw. 2017. "Role of Vitamin D in Maintaining Renal Epithelial Barrier Function in Uremic Conditions" International Journal of Molecular Sciences 18, no. 12: 2531. https://doi.org/10.3390/ijms18122531
APA StyleMihajlovic, M., Fedecostante, M., Oost, M. J., Steenhuis, S. K. P., Lentjes, E. G. W. M., Maitimu-Smeele, I., Janssen, M. J., Hilbrands, L. B., & Masereeuw, R. (2017). Role of Vitamin D in Maintaining Renal Epithelial Barrier Function in Uremic Conditions. International Journal of Molecular Sciences, 18(12), 2531. https://doi.org/10.3390/ijms18122531