1. Introduction
New probes for tumor imaging and local therapies are an important clinical need. Currently, clinical developments are mainly based on new radionuclides for positron emission tomography (PET). However, there is growing interest in nanoparticles (NPs), because they offer various specific properties, including high surface-to-volume ratio, high surface energy, and a wide range of additional mechanical, thermal, electrical, magnetic, and optical properties [
1,
2,
3]. NPs further offer possibilities to combine several contrast agents for multimodal imaging, to be decorated with various biological and chemical moieties and to cargo therapeutic agents.
Determining the distribution of nanocarriers within the body following systemic administration in order to achieve high accumulation of NPs in tumors with low background in other tissues is the major challenge for nanomedicine. NP characteristics have an impact on their pharmacokinetics [
4,
5], and longer plasmatic half-life favors higher accumulation within the tumor. Such accumulation of NPs in tumors results from different mechanisms, either specific or nonspecific, and involves different cell populations, including cancer and stroma cells [
6,
7].
The enhanced permeability and retention (EPR) effect [
8,
9] has been suggested to be the major underlying mechanism of passive NP accumulation in tumors. The EPR effect, although efficient in mice, appears to be extremely heterogeneous—or possibly totally ineffective—in humans [
10,
11], resulting in low or no accumulation of NPs in human tumors. Since the EPR effect fails in the clinic, new tumor models with low spontaneous NP accumulation are required to screen imaging probes and to test new targeting strategies.
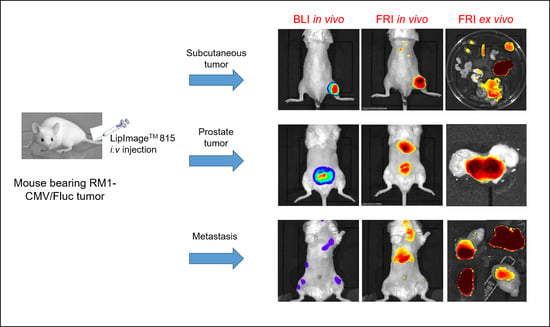
Fluorescent imaging on mice models is a convenient way to initiate the screening process of NP-based imaging probes and to provide key information about NP properties. Although in vivo fluorescence imaging does not discriminate between the different mechanisms involved in NP accumulation in tumors, it allows for rapid evaluation of the overall targeting efficiency [
12]. In order to develop new tumor models in mice, the aim of the present work is to study NP accumulation for a single tumor cell line according to different tumor locations. For this purpose, RM1, a murine prostate cancer cell line, was used at different implantation sites to generate subcutaneous, orthotopic and metastatic tumors. LipImage
TM 815, a non-specific nanosized (80-nm diameter) fluorescent imaging agent, was injected intravenously to compare NP accumulation in the various tumor locations and types.
3. Discussion
Using a single murine prostate cancer cell line (RM1), via different routes, to generate various types of tumors (orthotopic, subcutaneous and metastatic), a constant dose of NPs and a wide panel of in vivo and ex vivo fluorescence imaging techniques, we demonstrated here that the non-specific labeling pattern varied according to tumor type. LipImageTM 815 accumulation was highly predominant in orthotopic tumors when compared with subcutaneous tumors and disseminated metastasis.
RM1 is a mouse prostate cancer cell line syngenic in C57BL/6 mice [
13]. It was genetically modified to produce a constitutive Fluc reporter gene and to make in vivo detection by BLI possible, even for small or deep tumors in mice. The BLI signal was not only used to follow tumor growth, but also to compare BLI and fluorescence imaging patterns. As luciferase activity requires oxygen and intraperitoneal injected luciferin (M
W = 280 D) as a substrate, the BLI signal further confirms that the tumors are not hypoxic, and are properly vascularized. RM1 tumors are very fast-growing, providing significant orthotopic prostate tumors in 4 days, subcutaneous tumors in 7 days, and metastasis in 13 days. Thus, the present RM1 models provided a complete panel of different tumor types exhibiting different levels of NP accumulation, making wide screening of NPs possible in a short period of time. On the other hand, the rapid growth of RM1 tumors is not favorable for long term studies, as tumors rapidly reached ethical endpoints. Finally, as RM1 is syngenic in C57BL/6 mice, the tumors grew in a microenvironment more representative of the clinical physio-pathological context. The rapid growth of RM1 tumors is a convenient feature for experimental purposes, but clearly diverges from a human tumor growth rate that may influence tumor properties. In the current work, however, the level of NP accumulation in RM1 tumors is not correlated with growing time.
Present data showed that the level of NP accumulation in a tumor was not a characteristic of the tumor cells, but involved other parameters, including tumor location. As a limitation of the present study, the different tumor locations required different implantation methods, which may influence NP accumulation. For metastasis, however, the fluorescence level is low, irrespective of location.
The fluorescent signal in tumors may result from several mechanisms, including retention in extracellular space, binding to or internalization in tumor cells, and tumor microenvironment components [
6,
7]. Variations in NP accumulation in tumors are often attributed to variations in EPR effect. The EPR effect results from hyper-permeability of the tumor blood vessels and dysfunction of intra-tumoral lymphatics drainage; the first mechanism enables nanoparticles to enter the tumor interstitial space, while the second one allows particles to stay in the tumor for a longer time [
14]. In the present work, solid evidence that accumulation results from a true EPR effect is lacking. Vascularization is a key parameter for NP accumulation in different tumors types; but in the present work, no information—such as pericytes coverage or fenestration sizes—is available. Regardless of the mechanism involved, our results confirmed that the resulting non-specific accumulation of NPs in tumors is highly variable. Conversely, accumulation found in some other normal tissues demonstrates that these mechanisms are no more efficient within metastasis or subcutaneous tumors than in healthy tissues.
LipImage
TM 815 NPs (50 nm) have previously been characterized for their optical and pharmacokinetic characteristics [
15], and have been shown to display a long shelf life, as well as colloidal and optical stabilities, with high brightness and strong and long accumulation in subcutaneous tumors in mice [
15]. Injection of these NPs in immunodeficient Swiss nude mice with implanted human prostate cancer PC3 resulted in strong and long-term fluorescent labeling of the tumor [
15], but data are lacking concerning NP stability. LipImage
TM 815 NPs (80 nm) also accumulated in RM1 subcutaneous tumors at 24 h, but their long-term residence could not be confirmed, as tumors grew very quickly, requiring rapid euthanasia of the mice. The post-mortem analysis of individual organs excised 24 h post NP injection showed high accumulation in the liver, but the fluorescent signal in the tumor was about 4 times less, and was not clearly different from those in organs such as the kidneys, intestines or spleen. As subcutaneous tumors grew on the surface, fluorescence from NP accumulation was easily detected, and changes in the fluorescence signal could be quantified. That makes this model quite attractive, as small chemical modifications in NPs induced changes in NP accumulation in the tumor that could be easily monitored by FRI [
12].
RM1 orthotopic tumors are deep in the body and thus detection of the fluorescence signal was impaired by photon absorption by the tissues. However, a deep tumor location is more favorable for fluorescence tomography, and FMT allowed for fluorescence detection and absolute in-depth quantification. Both in vivo and ex vivo imaging methods confirmed high levels of accumulation of LipImageTM 815 in RM1 orthotopic tumors. Because they grow in an original microenvironment, orthotopic tumors are relevant from a physio-pathological point of view; but, as they exhibit a high level of passive NP accumulation, they behave quite differently from the clinical context.
LipImage
TM 815 accumulation in disseminated metastasis is low at 24 h. Combined with in-depth localization, the fluorescence signal is not detectable by in vivo fluorescence imaging. Tumor localization is made possible by BLI, and a fluorescence signal is detectable by ex vivo FRI only in organs with low background. The low level of LipImage
TM 815 accumulation in RM1 metastasis may be relevant for screening new strategies dedicated to circumventing EPR failures currently reported in a clinical context [
16].
The choice of animal model necessary for rapid and extensive evaluation of NPs requires improvement towards a more clinically-relevant model for imaging probe evaluation. As illustrated by this study using LipImageTM 815, even when using a single NP and a single cell line, non-specific accumulation clearly depends on a lot of factors, including tumor type and location. Other factors, such as tumor size, injection routes, doses and injection scheme, may further influence NP accumulation. Although it is not wholly representative of the clinical context, each tumor type may be useful for challenging NP properties.
4. Materials and Methods
4.1. Animal Handling and Tumor Generation
Animal manipulations, approved by the local ethical committee (CEEA 50) under agreement A50120196, were performed in agreement with French and European directives on the care and use of animals. B6 Albino (B6N-Tyrc−Brd/BrdCrCrl) mice (6- to 8-weeks-old) were maintained in standard conditions under a 12-h light/dark cycle with water and food provided ad libitum at the University of Bordeaux animal facilities. Manipulations were performed on anesthetized animals using 2% isoflurane (Belamont, Nicholas Piramal Limited, London, UK) in air.
Orthotopic tumors were induced by cell injection within the prostate on anesthetized mice. The skin and the abdominal muscles were incised by a short section and the seminal glands were pulled back outside the body. Cells (5 × 105/10 µL per lobe) were injected in the two dorsal prostate lobes and the seminal glands returned to the abdomen. The incision was then closed with sutures. Metastasis was induced by intra-cardiac cell injection (1 × 105/100 µL) in the left ventricle with ultrasound guidance on anesthetized mice. Subcutaneous tumors were generated by cell injection (2 × 106/100 µL) in the posterior right leg. Before the imaging session, regions to be imaged were shaved with clippers and depilatory cream. LipImageTM 815 (31.5 µM of NIR fluorophore) were injected via the tail vein. After in vivo imaging, organs were removed from euthanized mice and placed in cold phosphate-buffered saline (PBS) in a petri dish and imaged ex vivo.
4.2. LipImageTM 815 Synthesis and Characterization
The IR780-lipid dye was first synthetized [
15]. An oil premix with, respectively, 85, 255, and 65 mg of oil, Suppocire NB™ (Gattefosse S.A., Saint-Priest, France) and lecithin was prepared. IR780-lipid dye solution (10 mg/mL; 200 μL) in ethanol was poured into a 5-mL vial and mixed with the oil premix melted at 50 °C. The mixture was homogenized and the solvent was then evaporated under argon flux. After homogenization at 50 °C, the continuous aqueous phase, composed of 345 mg of Myrj
TM S40 (Croda Uniquema; Chocques, France) and the appropriate amount of aqueous solution (154 mM NaCl qs 2 mL), was introduced. The mixture was placed in a water bath at 50 °C and was then sonicated for 5 min using a VCX750 Ultrasonic processor (power output 190 W, 3-mm probe diameter, Sonics). LipImage
TM 815 solution was dialyzed against 1000 times their volume in the appropriate aqueous buffer overnight at room temperature (12 to 14,000 Da M
W cut off membranes, ZelluTrans, Carl Roth, France). The nanoparticle dispersion was finally sterilized by filtration through a 0.22 μm Millipore membrane. Size distribution of LipImage™ 815 was measured with a Zetasizer (Nano ZS, Malvern instrument, Worcestershire, UK) (
Figure S2). The number of particles was calculated by dividing the total volume of lipids (total mass of oil, wax, lecithin and PEG assuming an overall density of 1.05 g·cm
−3) divided by the individual volume of LipImage
TM 815 nanoparticles (size = 74.4 nm;
Figure S2).
4.3. Cell Line Generation and Culture
Murine prostate cancer cell line RM1, initially obtained from Dr. T.C. Thompson (Baylor College of Medicine, Houston, TX, USA), was genetically engineered for constitutive expression of firefly luciferase (RM1-CMV/Fluc) as previously described [
12], and was maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen), 1% antimycotic-antibiotic mix (PSA, Invitrogen) and blasticidin (10 µg/mL, Euromedex, Souffelweyersheim, France). Cell line was maintained in a humidified 5% CO
2 incubator at 37 °C.
4.4. Bioluminescence Imaging (BLI)
BLI was performed at Vivoptic (UMS 3767—Univ. Bordeaux) using Lumina LT (Perkin Elmer Inc., Boston, MA, USA). Mice received an intra-peritoneal injection of d-luciferin (2.9 mg in 100 µL PBS, Promega, Madison, WI, USA), and were anesthetized 5 min later. Bioluminescence images (1 min, 4 × 4 binning) and photographs (100 ms) were acquired successively 8 min after d-luciferin injection. Images acquisition and analysis were performed using Living Image software.
4.5. Fluorescence Reflectance Imaging (FRI)
FRI was performed using the Lumina LT apparatus (Perkin Elmer, Boston, MA, USA) with the 745 nm excitation filter and the 810–875 nm emission filter. Fluorescence images (1 s, 4 × 4 binning) and photographs (100 ms) were acquired successively, and analyzed using Living Image software. FRI signal is expressed as photons·s−1·cm−2·sr−1.
FRI was also performed using the per-operatory camera system Fluobeam® (Fluoptics, Grenoble, France) at a spectral window of excitation of 780 nm and with an emission of 820 nm. The image was analyzed using Image J software.
4.6. Fluorescence Molecular Tomography (FMT)
Mice were imaged in a Fluorescence Molecular Tomograph (FMT®) 4000 (Perkin Elmer, Boston, MA, USA). Scanning was performed using the 745 channel, and the fluorescence signal was filtered with the 770–800 nm filter emission. The images were reconstructed and analyzed using the TrueQuant software.
4.7. Histology and Microscopic Imaging
Tumors were frozen and stored at −80 °C. Tumor slices (10 µm) were obtained, fixed with 4% paraformaldehyde (10 min, room temperature), and hematoxylin-eosin-safran staining was performed. LipImageTM 815 fluorescence detection was performed using Leica DM 5500 microscope fitted with pE-100 (Ex 770 nm) Cool LED and a indocyanine (775/845 nm) filter (Leica Microsystems, Wetzlar, Germany).