Aberrant N-Glycosylation Profile of Serum Immunoglobulins is a Diagnostic Biomarker of Urothelial Carcinomas
Abstract
:1. Introduction
2. Results
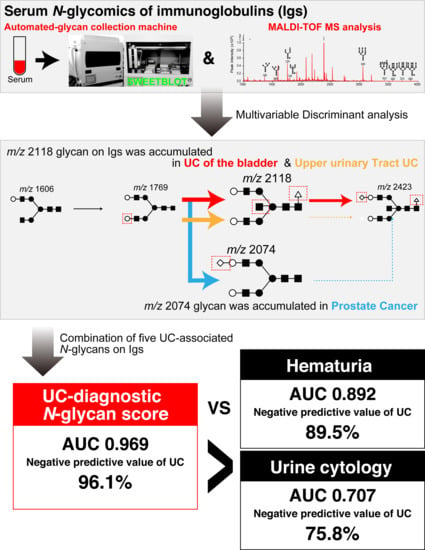
2.1. Downregulation of Asialo Biantennary Type N-Glycans and Accumulation of Asialo-Bisecting GlcNAc with Core Fucosylated N-Glycan on Igs May Occur as a UC-Associated Aberrant N-Glycosylation of Igs
2.2. UC Diagnostic N-Glycan Score Based on Aberrant N-Glycosylation of Igs Was Far Superior to Classic Urine Cytology and Hematuria Status
3. Discussion
4. Materials and Methods
4.1. Serum Samples
4.2. Purification and Quantification of the Igs Fraction from Serum
4.3. Serum N-Glycomics of Igs Performed by Using the Glycoblotting Method and Mass Spectorometric Analysis
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| UC | Urothelial carcinoma |
| UCB | Urothelial carcinoma of the bladder |
| UTUC | Upper urinary tract urothelial carcinoma |
| HV | Healthy volunteers |
| PC | Prostate cancer |
| BPH | Beneign prostatic hyperplasia |
| HSPC | Hormone sensitive prostate cancer |
| NMIBC | Nonmuscle invasive bladder cancer |
| MIBC | Muscle invasive bladder cancer |
| NI-UTUC | Noninvasive UTUC |
| I-UTUC | Invasive UTUC |
| Igs | Immunogloblins |
| dNGScore | Diagnostic N-glycan Score |
| AUC | Area under the curve |
| ROC | Receiver operating characteristic curves |
| BTA | Bladder tumor antigen |
| NMP22 | Nuclear matrix protein number 22 |
| IQR | Interquartile range |
| NPV | Negative predictive value |
| PPV | Positive predictive value |
| UTI | Urinary tract infection |
| MALDI-TOF | Matrix-assisted laser desorption time-of-flight |
| Man | Mannose |
| Gal | Galactose |
| GlcNAc | N-acetylglucosamine |
| Fuc | Fucose |
| Sia | N-acetylneuraminic acid |
| CBB | Coomassie brilliant blue |
Appendix A

| # | m/z | Composition |
|---|---|---|
| 1 | 1362.5 | (Hex)2 + (Man)3(GlcNAc)2 |
| 2 | 1524.5 | (Hex)3 + (Man)3(GlcNAc)2 |
| 3 | 1565.5 | (Hex)5 + (HexNAc)3 |
| 4 | 1590.6 | (HexNAc)2(dHex)1 + (Man)3(GlcNAc)2 |
| 5 | 1606.6 | (Hex)1(HexNAc)2 + (Man)3(GlcNAc)2 |
| 6 | 1647.6 | (HexNAc)3 + (Man)3(GlcNAc)2 |
| 7 | 1686.6 | (Hex)4 + (Man)3(GlcNAc)2 |
| 8 | 1708.6 | (Hex)1(HexNAc)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 9 | 1752.6 | (Hex)1(HexNAc)2(dHex)1 + (Man)3(GlcNAc)2 |
| 10 | 1768.6 | (Hex)2(HexNAc)2 + (Man)3(GlcNAc)2 |
| 11 | 1793.7 | (HexNAc)3(dHex)1 + (Man)3(GlcNAc)2 |
| 12 | 1809.7 | (Hex)1(HexNAc)3 + (Man)3(GlcNAc)2 |
| 13 | 1848.6 | (Hex)5 + (Man)3(GlcNAc)2 |
| 14 | 1870.7 | (Hex)2(HexNAc)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 15 | 1914.7 | (Hex)2(HexNAc)2(dHex)1 + (Man)3(GlcNAc)2 |
| 16 | 1955.7 | (Hex)1(HexNAc)3(dHex)1 + (Man)3(GlcNAc)2 |
| 17 | 2010.7 | (Hex)6 + (Man)3(GlcNAc)2 |
| 18 | 2032.7 | (Hex)3(HexNac)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 19 | 2057.8 | (Hex)1(HexNAc)2(dHex)1(NeuAc)1+ (Man)3(GlcNAc)2 |
| 20 | 2073.8 | (Hex)2(HexNAc)2(NeuAc)1+ (Man)3(GlcNAc)2 |
| 21 | 2117.8 | (Hex)2(HexNAc)3(dHex)1 + (Man)3(GlcNAc)2 |
| 22 | 2219.8 | (Hex)2(HexNAc)2(dHex)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 23 | 2336.9 | (Hex)3(HexNAc)4 + (Man)3(GlcNAc)2 |
| IS | 2348.9 1 | Internal standard (BOA-labeled A2 amide) |
| 24 | 2378.9 | (Hex)2(HexNAc)2(NeuAc)2 + (Man)3(GlcNAc)2 |
| 25 | 2422.9 | (Hex)2(HexNAc)3(dHex)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 26 | 2524.9 | (Hex)2(HexNAc)2(dHex)1(NeuAc)2 + (Man)3(GlcNAc)2 |
| 27 | 2727.9 | (Hex)2(HexNAc)3(dHex)1(NeuAc)2 + (Man)3(GlcNAc)2 |
| 28 | 2743.9 | (Hex)3(HexNAc)3(NeuAc)2 + (Man)3(GlcNAc)2 |
| 29 | 2890.1 | (Hex)3(HexNAc)3(dHex)1(NeuAc)2 + (Man)3(GlcNAc)2 |
| 30 | 3049.1 | (Hex)3(HexNAc)3(NeuAc)3 + (Man)3(GlcNAc)2 |
| 31 | 3195.2 | (Hex)3(HexNAc)3(dHex)1(NeuAc)3 + (Man)3(GlcNAc)2 |
| 32 | 3341.2 | (Hex)3 (HexNAc)3 (Deoxyhexose)2 (NeuAc)3 + (Man)3(GlcNAc)2 |
References
- Park, J.C.; Hahn, N.M. Bladder cancer: A disease ripe for major advances. Clin. Adv. Hematol. Oncol. 2014, 12, 838–845. [Google Scholar] [PubMed]
- Munoz, J.J.; Ellison, L.M. Upper tract urothelial neoplasms: Incidence and survival during the last 2 decades. J. Urol. 2000, 164, 1523–1525. [Google Scholar] [CrossRef]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Inman, B.A.; Tran, V.T.; Fradet, Y.; Lacombe, L. Carcinoma of the upper urinary tract: Predictors of survival and competing causes of mortality. Cancer 2009, 115, 2853–2862. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N.C. CT urography for hematuria. Nat. Rev. Urol. 2012, 9, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Bohle, A.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Hernandez, V.; Kaasinen, E.; Palou, J.; Roupret, M.; et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2016, 71, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.D.; Wood, C.G. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009, 115, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Ramakumar, S.; Bhuiyan, J.; Besse, J.A.; Roberts, S.G.; Wollan, P.C.; Blute, M.L.; O’Kane, D.J. Comparison of screening methods in the detection of bladder cancer. J. Urol. 1999, 161, 388–394. [Google Scholar] [CrossRef]
- Narayan, V.M.; Adejoro, O.; Schwartz, I.; Ziegelmann, M.; Elliott, S.; Konety, B.R. The Prevalence and Impact of Urinary Marker Testing in Patients with Bladder Cancer. J. Urol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, A.; Longo, T.A.; Fantony, J.J.; Owusu, R.; Foo, W.C.; Dash, R.; Inman, B.A. The diagnostic accuracy of urine-based tests for bladder cancer varies greatly by patient. BMC Urol. 2016, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S.; Amano, M.; Tobisawa, Y.; Yoneyama, T.; Tsuchiya, N.; Habuchi, T.; Nishimura, S.-I.; Ohyama, C. Serum N-Glycan Alteration Associated with Renal Cell Carcinoma Detected by High Throughput Glycan Analysis. J. Urol. 2014, 191, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Tobisawa, Y.; Hatakeyama, S.; Ohashi, T.; Tanaka, M.; Narita, S.; Koie, T.; Habuchi, T.; Nishimura, S.-I.; Ohyama, C.; et al. Serum tri- and tetra-antennary N-glycan is a potential predictive biomarker for castration-resistant prostate cancer. Prostate 2014, 74, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Maho Amano, M.T. N- and O-glycome analysis of serum and urine from bladder cancer patients using a high-throughput glycoblotting method. J. Glycom. Lipidom. 2013, 3, 108. [Google Scholar] [CrossRef]
- Narita, T.; Hatakeyama, S.; Yoneyama, T.; Narita, S.; Yamashita, S.; Mitsuzuka, K.; Sakurai, T.; Kawamura, S.; Tochigi, T.; Takahashi, I.; et al. Clinical implications of serum N-glycan profiling as a diagnostic and prognostic biomarker in germ-cell tumors. Cancer Med. 2017, 6, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Nouso, K.; Amano, M.; Ito, Y.M.; Miyahara, K.; Morimoto, Y.; Kato, H.; Tsutsumi, K.; Tomoda, T.; Yamamoto, N.; Nakamura, S.; et al. Clinical utility of high-throughput glycome analysis in patients with pancreatic cancer. J. Gastroenterol. 2013, 48, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, A.; Utratna, M.; O’Dwyer, M.E.; Joshi, L.; Kilcoyne, M. Glycosylation-Based Serum Biomarkers for Cancer Diagnostics and Prognostics. Biomed. Res. Int. 2015, 2015, 490531. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Lim, S.O.; Xia, W.; Lee, H.H.; Chan, L.C.; Kuo, C.W.; Khoo, K.H.; Chang, S.S.; Cha, J.H.; Kim, T.; et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016, 7, 12632. [Google Scholar] [CrossRef] [PubMed]
- Veillon, L.; Fakih, C.; Abou-El-Hassan, H.; Kobeissy, F.; Mechref, Y. Glycosylation Changes in Brain Cancer. ACS Chem. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, M.; Hatakeyama, S.; Yoneyma, T.; Tobisawa, Y.; Narita, T.; Yamamoto, H.; Hashimoto, Y.; Koie, T.; Narita, S.; Sasaki, A.; et al. Significance of Serum N-glycan Profiling as a Diagnostic Biomarker in Urothelial Carcinoma. Eur. Urol. Focus 2016. [Google Scholar] [CrossRef] [PubMed]
- Wuhrer, M.; Selman, M.H.; McDonnell, L.A.; Kumpfel, T.; Derfuss, T.; Khademi, M.; Olsson, T.; Hohlfeld, R.; Meinl, E.; Krumbholz, M. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J. Neuroinflamm. 2015, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Suzuki, H.; Makita, Y.; Takahata, A.; Takahashi, K.; Muto, M.; Sasaki, Y.; Kelimu, A.; Matsuzaki, K.; Yanagawa, H.; et al. Diagnosis and activity assessment of immunoglobulin A nephropathy: Current perspectives on noninvasive testing with aberrantly glycosylated immunoglobulin A-related biomarkers. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Malard-Castagnet, S.; Dugast, E.; Degauque, N.; Pallier, A.; Soulillou, J.P.; Cesbron, A.; Giral, M.; Harb, J.; Brouard, S. Sialylation of antibodies in kidney recipients with de novo donor specific antibody, with or without antibody mediated rejection. Hum. Immunol. 2015, 77, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, T.W.; Parekh, R.B.; Dwek, R.A.; Isenberg, D.; Rook, G.; Axford, J.S.; Roitt, I. The role of IgG glycoforms in the pathogenesis of rheumatoid arthritis. Springer Semin. Immunopathol. 1988, 10, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Kazuno, S.; Furukawa, J.; Shinohara, Y.; Murayama, K.; Fujime, M.; Ueno, T.; Fujimura, T. Glycosylation status of serum immunoglobulin G in patients with prostate diseases. Cancer Med. 2016, 5, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Ruhaak, L.R.; Barkauskas, D.A.; Torres, J.; Cooke, C.L.; Wu, L.D.; Stroble, C.; Ozcan, S.; Williams, C.C.; Camorlinga, M.; Rocke, D.M.; et al. The Serum Immunoglobulin G Glycosylation Signature of Gastric Cancer. EuPA Open Proteom. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Theodoratou, E.; Thaci, K.; Agakov, F.; Timofeeva, M.N.; Stambuk, J.; Pucic-Bakovic, M.; Vuckovic, F.; Orchard, P.; Agakova, A.; Din, F.V.; et al. Glycosylation of plasma IgG in colorectal cancer prognosis. Sci. Rep. 2016, 6, 28098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, B.; Wang, Y.; Xia, P.; He, C.; Liu, Y.; Zhang, R.; Zhang, M.; Li, Z. Disease-specific IgG Fc N-glycosylation as personalized biomarkers to differentiate gastric cancer from benign gastric diseases. Sci. Rep. 2016, 6, 25957. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Tobisawa, Y.; Kaya, T.; Kaneko, T.; Hatakeyama, S.; Mori, K.; Hashimoto, Y.; Koie, T.; Suda, Y.; Ohyama, C.; et al. Wisteria floribunda Agglutinin and Its Reactive-Glycan-Carrying Prostate-Specific Antigen as a Novel Diagnostic and Prognostic Marker of Prostate Cancer. Int. J. Mol. Sci. 2017, 18, 261. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hatakeyama, S.; Yamamoto, H.; Narita, T.; Hamano, I.; Matsumoto, T.; Soma, O.; Tobisawa, Y.; Yoneyama, T.; Yoneyama, T.; et al. Clinical relevance of aortic calcification in urolithiasis patients. BMC Urol. 2017, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- James, D.B.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Oxford, UK, 2016; p. 272. [Google Scholar]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Barkan, G.A.; Wojcik, E.M.; Nayar, R.; Savic-Prince, S.; Quek, M.L.; Kurtycz, D.F.; Rosenthal, D.L. The Paris System for Reporting Urinary Cytology: The Quest to Develop a Standardized Terminology. Adv. Anat. Pathol. 2016, 23, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S.; Amano, M.; Tobisawa, Y.; Yoneyama, T.; Tsushima, M.; Hirose, K.; Yoneyama, T.; Hashimoto, Y.; Koie, T.; Saitoh, H.; et al. Serum N-Glycan Profiling Predicts Prognosis in Patients Undergoing Hemodialysis. Sci. World J. 2013, 2013, 268407. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Christie, D. Resampling with Excel. Teach. Stat. 2004, 26, 9–14. [Google Scholar] [CrossRef]



| Non-UC a | UC b | p Value a vs. b | ||||
|---|---|---|---|---|---|---|
| HV | n, (%) | PC | n, (%) | n, (%) | ||
| Total patients (n) | 339 | 96 | 237 | |||
| Sex (Male, %) | 122 (36.0) | 96 (100) | 191 (80.6) | <0.001 | ||
| Median age (IQR 1) | 68.0 (63–73) | 74.0 (68–78) | 70.0 (62–75) | 0.700 | ||
| Former or current smoker | 75 (22.1) | 18 (18.8) | 71 (29.9) | 0.101 | ||
| Stone former | 4 (1.2) | 0 (0) | 0 (0) | 0.184 | ||
| BPH 2 | 7 (2.1) | 0 (0) | 0 (0) | 0.145 | ||
| HSPC 3 | 0 (0) | 96 (100) | 0 (0) | <0.001 | ||
| hematuria+ | 0 (0) | 0 (0) | 186 (78.5) | <0.001 | ||
| Urine Cytology Class | ||||||
| I, II | 81 (34.2) | |||||
| III | 58 (24.5) | |||||
| IV | 16 (6.7) | |||||
| V | 82 (34.6) | |||||
| Tumor Location of UC | ||||||
| Bladder | 177 (67.6) | |||||
| Renal pelvis | 27 (11.4) | |||||
| Ureter | 28 (11.8) | |||||
| Multiple | 4 (1.7) | |||||
| Tumor Grade of UC | ||||||
| Low grade noninvasive | 68 (28.7) | |||||
| High grade noninvasive | 43 (18.1) | |||||
| Muscle invasive | 109 (45.9) | |||||
| Lymph node stage N1 | 0 (0.0) | 20 (8.4) | 0.115 | |||
| Metastatic disease | 4 (4.2) | 47 (19.8) | 0.010 | |||
| Variables | Wilks’ Lambda | F Value | ODF 1 | TDF 2 | p Value | Discriminant Function |
|---|---|---|---|---|---|---|
| m/z 1606 | 0.9742 | 17.72 | 1 | 670 | <0.001 | 0.1925 |
| m/z 1769 | 0.9707 | 20.24 | 1 | 670 | <0.001 | 0.4932 |
| m/z 2074 | 0.9377 | 44.54 | 1 | 670 | <0.001 | 0.4941 |
| m/z 2118 | 0.5894 | 466.73 | 1 | 670 | <0.001 | ‒3.2460 |
| m/z 2423 | 0.9984 | 1.04 | 1 | 670 | <0.001 | 0.6179 |
| Constant term | ‒0.4905 | |||||
| Variables | AUC | 95% CI | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| Hematuria | 0.892 | 0.861–0.924 | 78.5 | 100.0 | 100.0 | 89.5 |
| Urine cytology | 0.707 | 0.661–0.753 | 41.4 | 100.0 | 100.0 | 75.8 |
| dNGScore 1 for UC | 0.969 | 0.952–0.986 | 92.8 | 97.2 | 94.8 | 96.1 |
| dNGScore for UCB | 0.993 | 0.982–0.100 | 98.3 | 97.2 | 93.5 | 99.3 |
| dNGScore for UTUC | 0.907 | 0.854–0.959 | 77.1 | 97.2 | 79.7 | 96.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.; Yoneyama, T.; Noro, D.; Imanishi, K.; Kojima, Y.; Hatakeyama, S.; Tobisawa, Y.; Mori, K.; Yamamoto, H.; Imai, A.; et al. Aberrant N-Glycosylation Profile of Serum Immunoglobulins is a Diagnostic Biomarker of Urothelial Carcinomas. Int. J. Mol. Sci. 2017, 18, 2632. https://doi.org/10.3390/ijms18122632
Tanaka T, Yoneyama T, Noro D, Imanishi K, Kojima Y, Hatakeyama S, Tobisawa Y, Mori K, Yamamoto H, Imai A, et al. Aberrant N-Glycosylation Profile of Serum Immunoglobulins is a Diagnostic Biomarker of Urothelial Carcinomas. International Journal of Molecular Sciences. 2017; 18(12):2632. https://doi.org/10.3390/ijms18122632
Chicago/Turabian StyleTanaka, Toshikazu, Tohru Yoneyama, Daisuke Noro, Kengo Imanishi, Yuta Kojima, Shingo Hatakeyama, Yuki Tobisawa, Kazuyuki Mori, Hayato Yamamoto, Atsushi Imai, and et al. 2017. "Aberrant N-Glycosylation Profile of Serum Immunoglobulins is a Diagnostic Biomarker of Urothelial Carcinomas" International Journal of Molecular Sciences 18, no. 12: 2632. https://doi.org/10.3390/ijms18122632
APA StyleTanaka, T., Yoneyama, T., Noro, D., Imanishi, K., Kojima, Y., Hatakeyama, S., Tobisawa, Y., Mori, K., Yamamoto, H., Imai, A., Yoneyama, T., Hashimoto, Y., Koie, T., Tanaka, M., Nishimura, S.-I., Kurauchi, S., Takahashi, I., & Ohyama, C. (2017). Aberrant N-Glycosylation Profile of Serum Immunoglobulins is a Diagnostic Biomarker of Urothelial Carcinomas. International Journal of Molecular Sciences, 18(12), 2632. https://doi.org/10.3390/ijms18122632