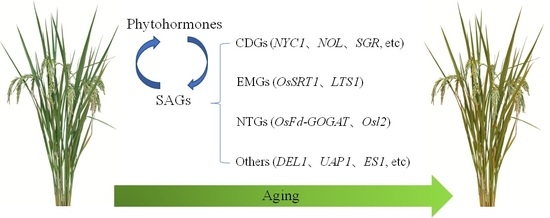
Genetic Dissection of Leaf Senescence in Rice
Abstract
:1. Introduction
2. Chloroplast Degradation Involved in Leaf Senescence
3. Phytohormones and Transcription Factors Involved in Rice Leaf Senescence
4. Energy Metabolism Pathway Regulated Rice Leaf Senescence
5. Nitrogen Remobilization Involved in Rice Leaf Senescence
6. Other Genes Involved in Leaf Senescence
7. Perspectives
Acknowledgments
Author Contributions
Conflicts of interest
Abbreviations
| ABA | abscisic acid |
| Chl | chlorophyll |
| ETH | ethylene |
| DGDG | digalactosyl diacylglycerol |
| GAPDH | glyceraldehyde-3-phosphatedehydrogenase |
| HG | homogalacturonan |
| H2O2 | hydrogen peroxide |
| JA | jasmonate |
| LHCⅡ | light-havesting complexⅡ |
| MeJA | methyl jasmonate |
| MGDG | monogalactosyl diacylglycerol |
| mGWAS | metabolite-based genome-wide association study |
| NAD | nicotinamide adenine dinucleotide |
| NADP | nicotinamide adenine dinucleotide phosphate |
| Nam | nicotinamide |
| NaMN | nicotinate mononucleotide |
| 1O2 | singlet oxygen |
| O2− | superoxide anion radical |
| OH | hydroxyl radical |
| PCD | programmed cell death |
| PEL | pectate lyase |
| PL | plasma membrane |
| PPH | pheophytinase |
| ROS | reactive oxygen species |
| SA | salicylic acid |
| SAGs | senescence-associated genes |
| SL | strigolactone |
| SSA | succinic semialdehyde |
| SSADH | succinic semialdehyde dehydrogenase |
| TCA | tricarboxylic acid |
| TFs | transcription factors |
References
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Quirino, B.F.; Noh, Y.S.; Himelblau, E.; Amasino, R.M. Molecular Aspect of Leaf Senescence. Trends Plant Sci. 2000, 5, 278–282. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Earl, S.; Harrison, E.; Mathas, E.; Navabpour, S.; Page, T.; Pink, D. The molecular analysis of leaf senescence—A genomics approach. Plant Biotechnol. J. 2003, 1, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Dani, K.G.S.; Fineschi, S.; Michelozzi, M.; Loreto, F. Do cytokinins, volatile isoprenoids and carotenoids synergically delay leaf senescence? Plant Cell Environ. 2016, 39, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Woo, R.H.; Nam, H.G. Toward systems understanding of leaf senescence: An integrated multi-omics perspective on leaf senescence research. Mol. Plant 2016, 9, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Schippers, J.H.M.; Schmidt, R.; Wagstaff, C.; Jing, H.C. Living to die and dying to live: The survival strategy behind leaf senescence. Plant Physiol. 2015, 169, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chang, C.; Tucker, M.L. To grow old: Regulatory role of ethylene and jasmonic acid in senescence. Front. Plant Sci. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schippers, J.H.M. Transcriptional networks in leaf senescence. Curr. Opin. Plant Biol. 2015, 27, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Buchanan-wollaston, V. The molecular biology of leaf senescence. J. Exp. Bot. 1997, 48, 181–199. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Y.; Zhou, G.; Ye, Z.J.; Zhao, L.N.; Li, X.H.; Lin, Y.J. Identification of early senescence-associated genes in rice flag leaves. Plant Mol. Biol. 2008, 67, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Li, Z.H.; Jiang, Z.Q.; Zhao, Y.; Peng, J.Y.; Jin, J.P.; Guo, H.W.; Luo, J.C. LSD: A leaf senescence database. Nucleic Acids Res. 2011, 39, D1103–D1107. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.D. Changes in chloroplast fine structure during the autumnal senescence of Betula leaves. Ann. Bot. 1970, 34, 817–824. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 2006, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Balazadeh, S.; Jaspert, N.; Arif, M.; Mueller-Roeber, B.; Maurina, V.G. Expression of ROS-responsive genes and transcription factors after metabolic formation of H2O2 in chloroplasts. Front. Plant Sci. 2012, 3, 234. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Li, M.R.; Chen, Y.P.; Wu, P.Z.; Wu, G.J.; Jiang, H.W. Knockdown of OsPAO and OsRCCR1 cause different plant death phenotypes in rice. J. Plant Physiol. 2011, 168, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, M.; Ito, H.; Morita, R.; Lida, S.; Sato, Y.; Fujimoto, M.; Kawasaki, S.; Tanaka, R.; Hirochik, H.; Nishimura, M.; et al. Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 2007, 19, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Morita, R.; Katsuma, S.; Nishimura, M.; Tanaka, A.; Kusaba, M. Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light-harvesting complex II degradation during senescence in rice. Plant J. 2009, 57, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Shioi, Y. Re-examination of Mg-dechelation reaction in the degradation of chlorophylls using chlorophyllin α as a substrate. Photosynth. Res. 2002, 74, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Morita, R.; Sato, Y.; Masuda, Y.; Nishimura, M.; Kusaba, M. Defect in non-yellow coloring 3, an α/β hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. Plant J. 2009, 59, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.W.; Li, M.R.; Liang, N.T.; Han, H.B.; Wei, Y.B.; Xu, X.L.; Liu, J.; Xu, Z.F.; Chen, F.; Wu, G.J. Molecular cloning and function analysis of the stay green gene in rice. Plant J. 2007, 52, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Yamatani, H.; Sato, Y.; Masuda, Y.; Kato, T.; Morita, R.; Fujunaga, K.; Nagamura, Y.; Nishimura, M.; Sakamoto, W.; Tanaka, A.; et al. NYC4, the rice ortholog of Arabidopsis THF1, is involved in the degradation of chlorophyll-protein complexes during leaf senescence. Plant J. 2013, 74, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Jiao, B.B.; Wang, J.J.; Zhu, X.D.; Zeng, L.J.; Li, Q.; He, Z.H. A novel protein RLS1 with NB-ARM domains is involved in chloroplast degradation during leaf senescence in rice. Mol. Plant 2012, 5, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Lin, M.C.; Chen, S.C.G. A novel alkaline α-galactosidase gene is involved in rice leaf senescence. Plant Mol. Biol. 2004, 55, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Chen, S.C.G. Programmed cell death during rice leaf senescence is non-apoptotic. New Phytol. 2002, 55, 25–32. [Google Scholar] [CrossRef]
- Uji, Y.; Akimitsu, K.; Gomi, K. Identification of OsMYC2-regulated senescence-associated genes in rice. Planta 2017, 245, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Sakuraba, Y.; Lee, T.; Kim, K.W.; An, G.; Lee, H.Y.; Paek, N.C. Mutation of Oryza sativa CORONATINE INSENSITIVE 1b (OsCOI1b) delays leaf senescence. J. Integr. Plant Biol. 2015, 57, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.Y.; Hua, Z.; Jian, W.; Wu, Y.Y.; Li, K.; Jin, C.; Chen, W.; Wang, S.C.; Wang, W.S.; Zhang, H.W.; et al. Control of leaf senescence by an MeOH-Jasmonates cascade that is epigenetically regulated by OsSRT1 in rice. Mol. Plant 2016, 9, 1366–1378. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Y.Y.; Luo, W.; Li, W.X.; Chen, N.; Zhang, D.J.; Chong, K. The F-box protein OsFBK12 targets OsSAMS1 for degradation and affects pleiotropic phenotypes, including leaf senescence, in Rice. Plant Physiol. 2013, 163, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Pan, G.; Li, K.Y.; Huang, F.D.; Cheng, F.M.; Pan, G. A single cytosine deletion in the OsPLS1 gene encoding vacuolar-type H+-ATPase subunit A1 leads to premature leaf senescence and seed dormancy in rice. J. Exp. Bot. 2016, 67, 2761–2776. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.W.; Casaretto, J.A.; Ying, S.; Mahmood, K.; Liu, F.; Bi, Y.M.; Rothstein, S.J. Overexpression of OsGATA12, regulates chlorophyll content, delays plant senescence and improves rice yield under high density planting. Plant Mol. Biol. 2017, 94, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Kim, C.Y.; Lee, J.; Lee, S.K.; Jeon, J.S. OsWRKY42 represses OsMT1d and induces rective oxygen species and leaf senescence in rice. Mol. Cells 2014, 37, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Luan, W.J.; Shen, A.; Jin, Z.P.; Song, S.S.; Long, L.Z.; Sha, A.H. Knockdown of OsHox33, a member of the class III homeodomain-leucine zipper gene family, accelerates leaf senescence in rice. Sci. China Life Sci. 2013, 56, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, S.; Piao, W.L.; Lim, J.H.; Han, S.H.; Kim, Y.S.; An, G.; Paek, N.C. Rice ONAC106 inhibits leaf senescence and increases salt tolerance and tiller angle. Plant Cell Physiol. 2015, 56, 2325–2339. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Z.; Wang, Y.Q.; Zhu, Y.N.; Tang, J.Y.; Hu, B.; Liu, L.C.; Ou, S.J.; Wu, H.K.; Sun, X.H.; Chu, J.F.; et al. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 10013–10018. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Yeung, E.; Bailey-Serres, J. The submergence tolerance gene SUB1A delays leaf senescence under prolonged darkness through hormonal regulation in rice. Plant Physiol. 2012, 160, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.S.; Li, M.N.; Yang, W.Q.; Xu, W.Y.; Xue, Y.B. A novel nuclear-localized CCCH-type zinc finger protein, OsDOS, is involved in delaying leaf senescence in rice. Plant Physiol. 2006, 141, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.; Maruyama, K.; Todaka, D.; Kidokoro, S.; Abo, M.; Yoshimura, E.; Shinozaki, K.; Nakashima, K.; Yamaguchi-Shinozaki, K. OsTZF1, a CCCH-tandem zinc finger protein, confers delayed senescence and stress tolerance in rice by regulating stress-related genes. Plant Physiol. 2013, 161, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.W.; Ren, D.Y.; Hu, S.K.; Li, G.M.; Dong, G.J.; Jiang, L.; Hu, X.M.; Ye, W.J.; Cui, Y.T.; Zhu, L.; et al. Down-Regulation of a Nicotinate Phosphoribosyltransferase Gene, OsNaPRT1, Leads to Withered Leaf Tips. Plant Physiol. 2016, 171, 1085–1098. [Google Scholar] [PubMed]
- Huang, L.M.; Sun, Q.W.; Qin, F.J.; Li, C.; Zhao, Y.; Zhou, D.X. Down-regulation of a SILENT INFORMATION REGULATOR2-related histone deacetylase gene, OsSRT1, induces DNA fragmentation and cell death in rice. Plant Physiol. 2007, 144, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.D.; Qin, R.; Li, M.; Alamin, M.; Jin, X.L.; Liu, Y.; Shi, C.H. The ferredoxin-dependent glutamate synthase (OsFd-GOGAT) participates in leaf senescence and the nitrogen remobilization in rice. Mol. Genet. Genom. 2016, 292, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.I.; Lee, R.H.; Chen, S.C.G. A novel senescence-associated gene encoding γ-aminobutyric acid (GABA):Pyruvate transaminase is upregulated during rice leaf senescence. Physiol. Plant. 2005, 123, 1–8. [Google Scholar] [CrossRef]
- Leng, Y.J.; Yang, Y.L.; Ren, D.Y.; Huang, L.C.; Dai, L.P.; Wang, Y.Q.; Chen, L.; Tu, Z.J.; Gao, Y.H.; Li, X.Y.; et al. A rice PECTATE LYASE-LIKE gene is required for plant growth and leaf senescence. Plant Physiol. 2017, 174, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Wang, Y.; Hong, X.; Hu, D.H.; Liu, C.X.; Yang, J.; Li, Y.; Huang, Y.Q.; Feng, Y.Q.; Gong, G.Y.; et al. Functional inactivation of UDP-N-acetylglucosamine pyrophosphorylase 1 (UAP1) induces early leaf senescence and defence responses in rice. J. Exp. Bot. 2015, 66, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.C.; Yang, Y.L.; Xu, J.; Li, X.J.; Leng, Y.J.; Dai, L.P.; Huang, L.C.; Shao, G.S.; Ren, D.Y.; Hu, J.; et al. EARLY SENESCENCE1 encodes a SCAR-LIKE PROTEIN2 that affects water loss in rice. Plant Physiol. 2015, 169, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, L.; Huang, W.F.; Yuan, M.; Zhou, F.; Li, X.H.; Lin, Y.J. Overexpression of OsSWEET5 in rice causes growth retardation and precocious senescence. PLoS ONE 2014, 9, e94210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.Y.; Yu, Q.; Wang, Z.Q.; Pan, Y.F.; Lv, W.T.; Zhu, L.L.; Chen, R.Z.; He, G.C. Knockdown of GDCH gene reveals reactive oxygen species-induced leaf senescence in rice. Plant Cell Environ. 2013, 36, 1476–1489. [Google Scholar] [CrossRef] [PubMed]
- Schulman, B.A.; Carrano, A.C.; Jeffrey, P.D.; Bowen, Z.; Kinnucan, E.R.; Finnin, M.S.; Elledge, S.J.; Harper, J.W.; Pagano, M.; Pavletich, N.P. Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature 2000, 408, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Furusawa, S.; Nagasaka, S.; Shimomura, K.; Yamaguchi, S.; Umehara, M. Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta 2014, 240, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Ariel, F.D.; Manavella, P.A.; Dezar, C.A.; Chan, R. The true story of the HD-Zip family. Trends Plant Sci. 2007, 12, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.A.; Park, J.H.; Lee, G.I.; Paek, K.H.; Park, S.K.; Nam, H.G. Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. Plant J. 1997, 12, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cai, Z.; Gan, S. Transcriptome of Arabidopsis leaf senescence. Plant Cell Environ. 2004, 27, 521–549. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC gene regulating senescence improves grain protein, Zinc, and iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar]
- Zhou, Y.; Huang, W.; Liu, L.; Chen, T.Y.; Zhou, F.; Lin, Y.J. Identification and functional characterization of a rice NAC gene involved in the regulation of leaf senescence. BMC Plant Biol. 2013, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Schommer, C.; Palatnik, J.F.; Aggarwal, P.; Chetelat, A.; Cubas, P.; Farmer, E.E.; Nath, U.; Weigel, D. Control of Jasmonate Biosynthesis and Senescence by miR319 Targets. PLoS Biol. 2008, 6, e230. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, W.; Hu, X.S.; Liu, H.J.; Lin, Y.J. W-box and G-box elements play important roles in early senescence of rice flag leaf. Sci. Rep. 2016, 6, 20881. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Ramirez-Hernandez, M.H.; Ziegler, M. The new life of a centenarian: Signalling functions of NAD(P). Trends Biochem. Sci. 2004, 29, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Queval, G.; Gakiere, B. NAD(P) synthesis and pyridine nucleotide cycling in plants and their potential importance in stress conditions. J. Exp. Bot. 2006, 57, 1603–1620. [Google Scholar] [CrossRef] [PubMed]
- Hashida, S.N.; Takahashi, H.; Uchimiya, H. The role of NAD biosynthesis in plant development and stress responses. Ann. Bot. 2009, 103, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Katoh, A.; Hashimoto, T. Molecular biology of pyridine nucleotide and nicotine biosynthesis. Front. Biosci. 2004, 9, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Pichersky, E. Nicotinamidase participates in the salvage pathway of NAD biosynthesis in Arabidopsis. Plant J. 2007, 49, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Muller, A.; Napoli, C.A.; Selinger, D.A.; Pikaard, C.S.; Richards, E.J.; Bender, J.; Mount, D.W.; Jorgensen, R.A. Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 2002, 30, 5036–5055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Zhou, D.X. Rice NAD+-dependent histone deacetylase OsSRT1 represses glycolysis and regulates the moonlighting function of GAPDH as a transcriptional activator of glycolytic genes. Nucleic Acids Res. 2017, 45. [Google Scholar] [CrossRef] [PubMed]
- Feller, U.; Fischer, A. Nitrogen metabolism in senescing leaves. Crit. Rev. Plant Sci. 1994, 13, 241–273. [Google Scholar] [CrossRef]
- Ansari, M.I.; Chen, S.C.G. Biochemical characterization of gamma-aminobutyric acid (GABA):Pyruvate transaminase during rice leaf senescence. Int. J. Integr. Biol. 2009, 6, 27–32. [Google Scholar]
- Ishizaki, T.; Ohsumi, C.; Totsuka, K.; Igarashi, D. Analysis of glutamate homeostasis by overexpression of Fd-GOGAT gene in Arabidopsis thaliana. Amino Acids 2010, 38, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Bown, A.W.; Shelp, B.J. The metabolism and function of gamma-aminobutyric acid. Plant Physiol. 1997, 115, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Svitkina, T.M.; Borisy, G.G. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 1999, 145, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Eitzen, G.; Wang, L.; Thorngren, N.; Wickner, W. Remodeling of organelle-bound actin is required for yeast vacuole fusion. J. Cell Biol. 2002, 158, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Sun, Y.; Drubin, D.G. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 2003, 115, 475–487. [Google Scholar] [CrossRef]
- Mathur, J.; Mathur, N.; Kernebeck, B.; Hulskamp, M. Mutations in actin-related proteins 2 and 3 affect cell shape development in Arabidopsis. Plant Cell 2003, 15, 1632–1645. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Lacomis, L.; Erdjument-Bromage, H.; Tempst, P.; Stamnes, M. Cytosol-derived proteins are sufficient for Arp2/3 recruitment and ARF/coatomer-dependent actin polymerization on Golgi membranes. FEBS Lett. 2004, 566, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Mallery, E.L.; Schlueter, J.; Huang, S.; Fan, Y.; Brankle, S.; Staiger, C.J.; Szymanski, D.B. Arabidopsis SCARs function interchangeably to meet actin-related protein 2/3 activation thresholds during morphogenesis. Plant Cell 2008, 20, 995–1011. [Google Scholar] [CrossRef] [PubMed]
- Engel, N.; van den Daele, K.; Kolukisaoglu, U.; Morgenthal, K.; Weckwerth, W.; Parnik, T.; Keerberg, O.; Bauwe, H. Deletion of glycine decarboxylase in Arabidopsis is lethal under nonphotorespiratory conditions. Plant Physiol. 2007, 144, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Lu, Q.T.; Yan, Y.; Ding, S.H.; Wen, X.G.; Lu, C.M. Comparative proteomic analysis provides new insights into the regulation of carbon metabolism during leaf senescence of rice grown under field conditions. J. Plant Physiol. 2010, 167, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.L.; Tian, Z.X.; Rao, Y.C.; Dong, G.J.; Yang, Y.L.; Huang, L.C.; Leng, Y.J.; Xu, J.; Sun, C.; Zhang, G.H.; et al. Rational design of high-yield and superior-quality rice. Nat. Plants 2017, 3, 17031. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.P. Development of hybrid rice to ensure food security. Rice Sci. 2014, 21, 1–2. [Google Scholar] [CrossRef]
- Thomas, H.; Ougham, H. The stay-green trait. J. Exp. Bot. 2014, 65, 3889–3900. [Google Scholar] [CrossRef] [PubMed]


| Gene | Accession Number | Functional Annotation | Mutant Phenotype | Overexpression Phenotype | Regulatory Role Δ | Ref. |
|---|---|---|---|---|---|---|
| OsPAO | LOC_Os03g05310 | Pheophorbide a oxygenase | early | unknown | − | [16] |
| OsRCCR1 | LOC_Os10g25030 | Red chlorophyll catabolite reductase | early | unknown | − | [16] |
| NYC1 | LOC_Os01g12710 | Short-chain dehydrogenase/reductase | delayed | unknown | + | [17] |
| NOL | LOC_Os03g45194 | Short-chain dehydrogenase/reductase | delayed | unknown | + | [18] |
| NYC3 | LOC_Os06g24730 | α/β hydrolase-fold family protein | delayed | unknown | + | [20] |
| SGR | LOC_Os09g36200 | Senescence-inducible chloroplast stay-green protein 1 | delayed | early | + | [21] |
| NYC4 | LOC_Os07g37250 | THYLAKOID FORMATION 1 | delayed | unknown | + | [22] |
| RLS1 | LOC_Os02g10900 | NB-ARC domain containing protein | early | early | − | [23] |
| Osh69 | LOC_Os08g38710 | Alkaline α-galactosidase | unknown | unknown | + | [24] |
| OsMYC2 | LOC_Os10g42430 | JA-inducible basic helix-loop-helix transcription factor | unknown | early | + | [26] |
| OsCOI1b | LOC_Os05g37690 | F-box domain and LRR containing protein | delayed | unknown | + | [27] |
| OsPME1 | LOC_Os01g57854 | Pectinesterase | delayed | early | + | [28] |
| OsTSD2 | LOC_Os02g51860 | Pectin methyltransferase | delayed | unknown | + | [28] |
| OsFBK12 | LOC_Os03g07530 | F-box protein containing a kelch repeat motif | early | delayed | − | [29] |
| OsPLS1 | LOC_Os06g45120 | Vacuolar H+-ATPase subunit A1 | early | unknown | − | [30] |
| OsGATA12 | LOC_Os03g61570 | GATA-like zinc finger transcription factor | unknown | delayed | − | [31] |
| OsWRKY42 | LOC_Os02g26430 | Nuclear transcriptional repressor | unknown | early | + | [32] |
| OsHox33 | LOC_Os12g41860 | Class III homeodomain-leucine zipper gene family | early | unknown | − | [33] |
| ONAC106 | LOC_Os01g66120 | NAC domain transcription factor | delayed | unknown | + | [34] |
| OsNAP/PS1 | LOC_Os03g21060 | No apical meristem | delayed | early | + | [35] |
| SUB1A * | No | Submergence tolerance regulator | unknown | delayed | − | [36] |
| OsDOS | LOC_Os01g09620 | Nuclear-localized CCCH-type zinc finger protein | early | delayed | − | [37] |
| OsTZF1 | LOC_Os05g10670 | CCCH-tandem zinc finger protein | early | delayed | − | [38] |
| LTS1/OsNaPRT1 | LOC_Os03g62110 | Nicotinate phosphoribosyltransferase | early | unknown | − | [39] |
| OsSRT1 | LOC_Os04g20270 | NAD+-dependent histone deacetylases | early | delayed | − | [40] |
| OsFd-GOGAT | LOC_Os07g46460 | Ferredoxin-dependent glutamate synthase | early | unknown | − | [41] |
| Osl2 | LOC_Os04g52450 | γ-aminobutyric acid (GABA):pyruvate transaminase | unknown | unknown | + | [42] |
| DEL1 | LOC_Os10g31910 | Pectate lyase precursor | early | unknown | − | [43] |
| SPL29 | LOC_Os08g10600 | UDP-N-acetylglucosamine pyrophosphorylase 1 | early | unknown | − | [44] |
| ES1/TUTOU1 | LOC_Os01g11040 | SCAR-like protein 2 | early | unknown | − | [45] |
| OsSWEET5 | LOC_Os05g51090 | Sugar transporter family | unknown | early | + | [46] |
| OsGDCH | LOC_Os10g37180 | Glycine decarboxylase complex H subunit | early | Unknown | − | [47] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, Y.; Ye, G.; Zeng, D. Genetic Dissection of Leaf Senescence in Rice. Int. J. Mol. Sci. 2017, 18, 2686. https://doi.org/10.3390/ijms18122686
Leng Y, Ye G, Zeng D. Genetic Dissection of Leaf Senescence in Rice. International Journal of Molecular Sciences. 2017; 18(12):2686. https://doi.org/10.3390/ijms18122686
Chicago/Turabian StyleLeng, Yujia, Guoyou Ye, and Dali Zeng. 2017. "Genetic Dissection of Leaf Senescence in Rice" International Journal of Molecular Sciences 18, no. 12: 2686. https://doi.org/10.3390/ijms18122686
APA StyleLeng, Y., Ye, G., & Zeng, D. (2017). Genetic Dissection of Leaf Senescence in Rice. International Journal of Molecular Sciences, 18(12), 2686. https://doi.org/10.3390/ijms18122686