Differential Expression of VvLOXA Diversifies C6 Volatile Profiles in Some Vitis vinifera Table Grape Cultivars
Abstract
:1. Introduction
2. Results and Discussion
2.1. C6 Volatile Level, Odor Contribution, and Cultivar and Vintage Effect
2.2. Evolution of C6 Volatiles in Grapes during Berry Development
2.3. Transcript Level of Key LOX-HPL Pathway Genes during Development
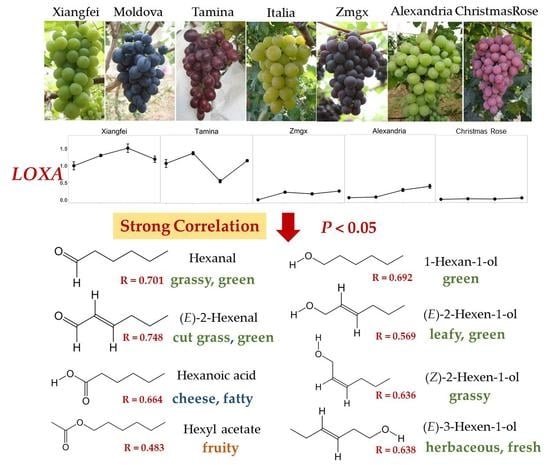
2.4. Correlation of Genes and C6 Volatiles
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Standards
4.2. Sample Collection
4.3. Extraction of Volatiles
4.4. Identification and Quantitation of Volatiles
4.5. Total RNA Extraction and Real-Time qPCR Assay
4.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Zmgx | Zaomeiguixiang |
| Alexandria | Muscat of Alexandria |
| OTV | Odor threshold value |
References
- Food and Agriculture Organization of the United Nations (FAO). Available online: http://faostat.fao.org/site/567/default.aspx (accessed on 17 May 2017).
- China Agriculture Research System for National Technology System for Grape Industry. In Research on the Sustainable Development Strategy of Modern Agricultural Industry in China—Grape Volume, 1st ed.; China Agriculture Press: Beijing, China, 2017; pp. 1–200.
- Carreno, I.; Cabezas, J.A.; Martinez-Mora, C.; Arroyo-Garcia, R.; Cenis, J.L.; Martinez-Zapater, J.M.; Carreno, J.; Ruiz-Garcia, L. Quantitative genetic analysis of berry firmness in table grape (Vitis vinifera L.). Tree Genet. Genomes 2015, 11, 818. [Google Scholar] [CrossRef]
- Lijavetzky, D.; Ruiz-Garcia, L.; Cabezas, J.A.; De Andres, M.T.; Bravo, G.; Ibanez, A.; Carreno, J.; Cabello, F.; Ibanez, J.; Martinez-Zapater, J.M. Molecular genetics of berry colour variation in table grape. Mol. Genet. Genom. 2006, 276, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Fournier-Level, A.; Lacombe, T.; Le Cunff, L.; Boursiquot, J.M.; This, P. Evolution of the VvMybA gene family, the major determinant of berry colour in cultivated grapevine (Vitis vinifera L.). Heredity 2010, 104, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Lasram, S.; Oueslati, S.; Mliki, A.; Ghorbel, A.; Silar, P.; Chebil, S. Ochratoxin a and ochratoxigenic black aspergillus species in tunisian grapes cultivated in different geographic areas. Food Control 2012, 25, 75–80. [Google Scholar] [CrossRef]
- Faci, J.M.; Blanco, O.; Medina, E.T.; Martinez-Cob, A. Effect of post veraison regulated deficit irrigation in production and berry quality of autumn royal and crimson table grape cultivars. Agric. Water Manag. 2014, 134, 73–83. [Google Scholar] [CrossRef]
- Zhu, S.; Liang, Y.; Gao, D.; An, X.; Kong, F. Spraying foliar selenium fertilizer on quality of table grape (Vitis vinifera L.) from different source varieties. Sci. Hortic. 2017, 218, 87–94. [Google Scholar] [CrossRef]
- Servili, A.; Feliziani, E.; Romanazzi, G. Exposure to volatiles of essential oils alone or under hypobaric treatment to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2017, 133, 36–40. [Google Scholar] [CrossRef]
- Walse, S.S.; Tebbets, J.S.; Leesch, J.G. Postharvest fumigation of california table grapes with ozone to control western black widow spider (Araneae: Theridiidae). Postharvest Biol. Technol. 2017, 134, 1–4. [Google Scholar] [CrossRef]
- Delele, M.A.; Ngcobo, M.E.; Opara, U.L.; Meyer, C.J. Investigating the effects of table grape package components and stacking on airflow, heat and mass transfer using 3-D CFD modelling. Food Bioprocess Technol. 2013, 6, 2571–2585. [Google Scholar] [CrossRef]
- Liguori, G.; Sortino, G.; De Pasquale, C.; Inglese, P. Effects of modified atmosphere packaging on quality parameters of minimally processed table grapes during cold storage. Adv. Hortic. Sci. 2015, 29, 152–154. [Google Scholar]
- Wu, Y.S.; Duan, S.Y.; Zhao, L.P.; Gao, Z.; Luo, M.; Song, S.R.; Xu, W.P.; Zhang, C.X.; Ma, C.; Wang, S.P. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Liu, B.; Zhu, B.Q.; Lan, Y.B.; Gao, Y.; Wang, D.; Reeves, M.J.; Duan, C.Q. Differences in volatile profiles of Cabernet Sauvignon grapes grown in two distinct regions of China and their responses to weather conditions. Plant Physiol. Biochem. 2015, 89, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Garcia, L.; Hellin, P.; Flores, P.; Fenoll, J. Prediction of muscat aroma in table grape by analysis of rose oxide. Food Chem. 2014, 154, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Qian, M.C. Development of C13-norisoprenoids, carotenoids and other volatile compounds in Vitis vinifera L. Cv. Pinot noir grapes. Food Chem. 2016, 192, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.X.; Wang, Y.J.; Liang, Z.C.; Fan, P.G.; Wu, B.H.; Yang, L.; Wang, Y.N.; Li, S.H. Volatiles of grape berries evaluated at the germplasm level by headspace-SPME with GC-MS. Food Chem. 2009, 114, 1106–1114. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Evolution of volatile compounds during the development of Cabernet Sauvignon grapes (Vitis vinifera L.). J. Agric. Food Chem. 2009, 57, 3818–3830. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.Q.; He, F.; Zhu, B.Q.; Lan, Y.B.; Pan, Q.H.; Li, C.Y.; Reeves, M.J.; Wang, J. Free and glycosidically bound aroma compounds in cherry (Prunus avium L.). Food Chem. 2014, 152, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, X.X.; Jin, Q.; Yang, L.L.; Li, J.M.; Chen, F. Free and bound volatile chemicals in mulberry (Morus atropurpurea Roxb.). J. Food Sci. 2015, 80, C975–C982. [Google Scholar] [CrossRef] [PubMed]
- Calín-Sánchez, Á.; Martínez-Nicolás, J.J.; Munera-Picazo, S.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Bioactive compounds and sensory quality of black and white mulberries grown in spain. Plant Food. Hum. Nutr. 2013, 68, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Baldwin, E.A.; Imahori, Y.; Kostenyuk, I.; Burns, J.; Brecht, J.K. Chilling and heating may regulate C6 volatile aroma production by different mechanisms in tomato (Solanum lycopersicum) fruit. Postharvest Biol. Technol. 2011, 60, 111–120. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Kalua, C.M.; Boss, P.K. Comparison of major volatile compounds from Riesling and Cabernet Sauvignon grapes (Vitis vinifera L.) from fruitset to harvest. Aust. J. Grape Wine Res. 2010, 16, 337–348. [Google Scholar] [CrossRef]
- Wang, R.; Sun, Q.; Chang, Q. Soil types effect on grape and wine composition in helan mountain area of ningxia. PLoS ONE 2015, 10, e0116690. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Xu, X.-Q.; Yu, K.-J.; Zhu, B.-Q.; Lan, Y.-B.; Duan, C.-Q.; Pan, Q.-H. Varietal dependence of GLVs accumulation and LOX-HPL pathway gene expression in four Vitis vinifera wine grapes. Int. J. Mol. Sci. 2016, 17, 1924. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Cheng, G.; Duan, L.L.; Jiang, R.; Pan, Q.H.; Duan, C.Q.; Wang, J. Effect of training systems on fatty acids and their derived volatiles in Cabernet Sauvignon grapes and wines of the north foot of Mt. Tianshan. Food Chem. 2015, 181, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Merillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Diago, M.P.; Vilanova, M.; Tardaguila, J. Effects of timing of manual and mechanical early defoliation on the aroma of Vitis vinifera L. Tempranillo wine. Am. J. Enol. Vitic. 2010, 61, 382–391. [Google Scholar]
- Vilanova, M.; Diago, M.P.; Genisheva, Z.; Oliveira, J.M.; Tardaguila, J. Early leaf removal impact on volatile composition of Tempranillo wines. J. Sci. Food Agric. 2012, 92, 935–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Wang, Y.; Wu, B.; Fang, J.; Li, S. Volatile compounds evolution of three table grapes with different flavour during and after maturation. Food Chem. 2011, 128, 823–830. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Dennison, R.A.; Dougherty, R.H.; Shaw, P.E. Flavor and odor thresholds in water of selected orange juice components. J. Agric. Food Chem. 1978, 26, 187–191. [Google Scholar] [CrossRef]
- De Lerma, N.L.; Moreno, J.; Peinado, R. Determination of the optimum sun-drying time for Vitis vinifera L. Cv. Tempranillo grapes by e-nose analysis and characterization of their volatile composition. Food Bioprocess Technol. 2014, 7, 732–740. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Jiang, W.; Li, J. Identification and quantification of impact aroma compounds in 4 nonfloral Vitis vinifera varieties grapes. J. Food Sci. 2010, 75. [Google Scholar] [CrossRef] [PubMed]
- Jetti, R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M. Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 2007, 72, S487–S496. [Google Scholar] [CrossRef] [PubMed]
- Noguerol-Pato, R.; Gonzalez-Alvarez, M.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Simal-Gandara, J. Aroma profile of garnacha tintorera-based sweet wines by chromatographic and sensorial analyses. Food Chem. 2012, 134, 2313–2325. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Podolyan, A.; White, J.; Jordan, B.; Winefield, C. Identification of the lipoxygenase gene family from Vitis vinifera and biochemical characterisation of two 13-lipoxygenases expressed in grape berries of Sauvignon Blanc. Funct. Plant Biol. 2010, 37, 767–784. [Google Scholar] [CrossRef]
- Zhu, B.Q.; Xu, X.Q.; Wu, Y.W.; Duan, C.Q.; Pan, Q.H. Isolation and characterization of two hydroperoxide lyase genes from grape berries. Mol. Biol. Rep. 2012, 39, 7443–7455. [Google Scholar] [CrossRef] [PubMed]
- Tesniere, C.; Davies, C.; Sreekantan, L.; Bogs, J.; Thomas, M.; Torregrosa, L. Analysis of the transcript levels of VvAdh1, VvADH2 and VvGrip4, three genes highly expressed during Vitis vinifera L. Berry development. Vitis 2006, 45, 75–79. [Google Scholar]
- Dunlevy, J.D.; Soole, K.L.; Perkins, M.V.; Nicholson, E.L.; Maffei, S.M.; Boss, P.K. Determining the methoxypyrazine biosynthesis variables affected by light exposure and crop level in Cabernet Sauvignon. Am. J. Enol. Vitic. 2013, 64, 450–458. [Google Scholar] [CrossRef]
- Kobayashi, S.; Goto-Yamamoto, N.; Hirochika, H. Retrotransposon-induced mutations in grape skin color. Science 2004, 304, 982. [Google Scholar] [CrossRef] [PubMed]
- Azuma, A.; Ban, Y.; Sato, A.; Kono, A.; Shiraishi, M.; Yakushiji, H.; Kobayashi, S. Myb diplotypes at the color locus affect the ratios of tri/di-hydroxylated and methylated/non-methylated anthocyanins in grape berry skin. Tree Genet. Genomes 2015, 11, 31. [Google Scholar] [CrossRef]
- Coombe, B.G. Growth stages of the grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Lan, Y.-B.; Qian, X.; Yang, Z.-J.; Xiang, X.-F.; Yang, W.-X.; Liu, T.; Zhu, B.-Q.; Pan, Q.-H.; Duan, C.-Q. Striking changes in volatile profiles at sub-zero temperatures during over-ripening of ‘Beibinghong’grapes in northeastern China. Food Chem. 2016, 212, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.E.; Olsson, N.; Schlosser, J.; Peng, F.; Lund, S.T. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef] [PubMed]



| Volatiles | Cultivar | Vintage | Cultivar × Vintage | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F Value | p | Sig. | F Value | p | Sig. | F Value | p | Sig. | |
| Hexanal | 75.4 | 7.49 × 10−10 | *** | 468.3 | 3.68 × 10−12 | *** | 64.3 | 2.19 × 10−9 | *** |
| (E)-2-Hexenal | 359.68 | 1.65 × 10−14 | *** | 2215.01 | <2 × 10−16 | *** | 92.62 | 1.86 × 10−10 | *** |
| Total C6 aldehydes | 184.85 | 1.65 × 10−12 | *** | 1309.51 | 3.12 × 10−15 | *** | 49.74 | 1.20 × 10−8 | *** |
| 1-Hexanol | 12.81 | 5.56 × 10−5 | *** | 18.41 | 0.00075 | *** | 2.55 | 0.06964 | |
| (E)-2-Hexen-1-ol | 36.56 | 9.04 × 10−8 | *** | 51.22 | 4.88 × 10−6 | *** | 22.64 | 1.89 × 10−6 | *** |
| (Z)-2-Hexen-1-ol | 35.89 | 1.02 × 10−7 | *** | 598.84 | 6.87 × 10−13 | *** | 35.89 | 1.02 × 10−7 | *** |
| (E)-3-Hexen-1-ol | 23.64 | 1.44 × 10−6 | *** | 130.75 | 1.73 × 10−8 | *** | 16.62 | 1.23 × 10−5 | *** |
| (Z)-3-Hexen-1-ol | 707.9 | <2 × 10−16 | *** | 1833.2 | 3.02 × 10−16 | *** | 293.7 | 6.72 × 10−14 | *** |
| Total C6 alcohols | 34.15 | 1.40 × 10−7 | *** | 58.07 | 2.40 × 10−6 | *** | 19.84 | 4.24 × 10−6 | *** |
| Ethyl hexanoate | 2.49 | 0.07529 | 23.14 | 0.00028 | *** | 2.84 | 0.05024 | ||
| Hexyl acetate | 17.89 | 7.94 × 10−6 | *** | 1.3 | 0.274 | 6.79 | 0.00155 | ** | |
| (Z)-3-Hexenyl acetate | 189.51 | 1.39 × 10−12 | *** | 40.95 | 1.66 × 10−5 | *** | 25.98 | 8.01 × 10−7 | *** |
| Total C6 esters | 42.34 | 3.48 × 10−8 | *** | 3.3 | 0.09098 | 7.24 | 0.00114 | ** | |
| Hexanoic acid | 11.73 | 9.11 × 10−5 | *** | 78.22 | 4.18 × 10−7 | *** | 4.76 | 0.00764 | ** |
| Compound | AAT | ADH1 | ADH2 | HPL1 | LOXA | LOXO |
|---|---|---|---|---|---|---|
| Hexanal | 0.478 * | 0.525 * | 0.533 * | 0.701 ** | 0.529 * | |
| (E)-2-Hexenal | 0.748 ** | 0.766 ** | 0.496 * | 0.748 ** | ||
| Aldehydes | 0.685 ** | 0.720 ** | 0.570 ** | 0.804 ** | ||
| 1-Hexanol | 0.564 ** | 0.692 ** | ||||
| (E)-2-Hexen-1-ol | 0.668 ** | 0.569 ** | ||||
| (Z)-2-Hexen-1-ol | 0.805 ** | 0.636 ** | ||||
| (E)-3-Hexen-1-ol | 0.583 ** | 0.638 ** | ||||
| (Z)-3-Hexen-1-ol | −0.496 * | |||||
| Alcohols | 0.655 ** | 0.568 ** | ||||
| Ethyl hexanoate | 0.452 * | |||||
| Hexyl acetate | 0.757 ** | 0.483 * | ||||
| (Z)-3-Hexenyl acetate | ||||||
| Esters | ||||||
| Hexanoic acid | 0.772 ** | 0.564 ** | 0.509 * | 0.664 ** | 0.613 ** | |
| Total amount | 0.785 ** | 0.630 ** | 0.455 * | 0.792 ** |
| Cultivar | Color of Berry Skin | Country of Origin of the Variety | Pedigree as Given by Breeder/Bibliography | Breeder | Flavor Description |
|---|---|---|---|---|---|
| Xiangfei | Blanc | China | 73-7-6 (Muscat Hamburg × Pearl of Csaba) × Cardinal | Institute of Forestry and Pomology | strong muscat and green |
| Zaomeiguixiang | Rouge | China | Muscat Hamburg × Pearl of Csaba | Institute of Forestry and Pomology | strong muscat |
| Muscat of Alexandria | Blanc | Greece | Heptakilo × Muscat Blanc a Petits Grains | moderate muscat | |
| Tamina | Rouge | Romania | Bicane × Muscat Hamburg | Gorodea, Gr.; Boian, I.; Lumanare, Zamfiritra | muscat |
| Italia | Blanc | Italy | Bicane × Muscat Hamburg | Pirovano, Alberto | light muscat, sweet and green |
| Moldova | Noir | Moldova | Guzal Kara × S.V. 12-375 | Zhuravel, M.S.; Gavrilov, I.P.; Borzikova, G.M.; Guzun, N.I. | strong green |
| Christmas Rose | Rouge | USA | S44-35C × 9-117D | Olmo, Harold, P.; Koyama, Albert T. | light flavor |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Sun, L.; Xu, X.-Q.; Zhu, B.-Q.; Xu, H.-Y. Differential Expression of VvLOXA Diversifies C6 Volatile Profiles in Some Vitis vinifera Table Grape Cultivars. Int. J. Mol. Sci. 2017, 18, 2705. https://doi.org/10.3390/ijms18122705
Qian X, Sun L, Xu X-Q, Zhu B-Q, Xu H-Y. Differential Expression of VvLOXA Diversifies C6 Volatile Profiles in Some Vitis vinifera Table Grape Cultivars. International Journal of Molecular Sciences. 2017; 18(12):2705. https://doi.org/10.3390/ijms18122705
Chicago/Turabian StyleQian, Xu, Lei Sun, Xiao-Qing Xu, Bao-Qing Zhu, and Hai-Ying Xu. 2017. "Differential Expression of VvLOXA Diversifies C6 Volatile Profiles in Some Vitis vinifera Table Grape Cultivars" International Journal of Molecular Sciences 18, no. 12: 2705. https://doi.org/10.3390/ijms18122705
APA StyleQian, X., Sun, L., Xu, X. -Q., Zhu, B. -Q., & Xu, H. -Y. (2017). Differential Expression of VvLOXA Diversifies C6 Volatile Profiles in Some Vitis vinifera Table Grape Cultivars. International Journal of Molecular Sciences, 18(12), 2705. https://doi.org/10.3390/ijms18122705