Influence of Transgenic Metallothionein-1 on Gliosis, CA1 Neuronal Loss, and Brain Metal Levels of the Tg2576 Mouse Model of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
2.1. MT-1/2 Immunostaining Is Dramatically Increased in TgMT Mice
2.2. Mt1 Overexpression Has Only Minor Effects on the Gliosis Elicited by Amyloid Plaques
2.3. Mt1 Overexpression Does Not Affect Hippocampal CA1 Neuronal Loss
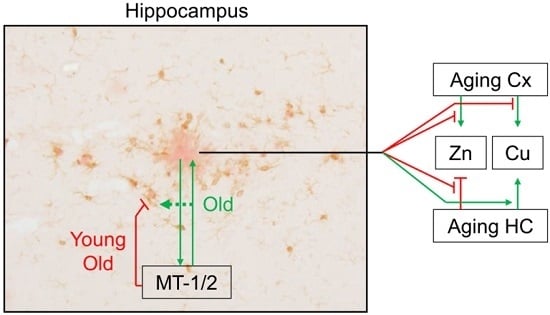
2.4. Mt1 Overexpression Has only Minor Effects on Zinc and Copper Levels
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Immunohistochemistry (IHC) and Histochemistry (HC)
4.3. Western Blotting
4.4. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bertram, L.; Lill, C.M.; Tanzi, R.E. The genetics of Alzheimer’s disease: Back to the future. Neuron 2010, 68, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Ittner, L.M.; Götz, J. Amyloid-β and tau—A toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I.; Pettingell, W.H.; Multhaup, G.; Paradis, M.d.; Vonsattel, J.P.; Gusella, J.F.; Beyreuther, K.; Masters, C.L.; Tanzi, R.E. Rapid induction of Alzheimer a beta amyloid formation by zinc. Science 1994, 265, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I. The metallobiology of Alzheimer’s disease. Trends Neurosci. 2003, 26, 207–214. [Google Scholar] [CrossRef]
- Duguid, J.R.; Bohmont, C.W.; Liu, N.G.; Tourtellotte, W.W. Changes in brain gene expression shared by scrapie and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 1989, 86, 7260–7264. [Google Scholar] [CrossRef] [PubMed]
- Zambenedetti, P.; Giordano, R.; Zatta, P. Metallothioneins are highly expressed in astrocytes and microcapillaries in Alzheimer’s disease. J. Chem. Neuroanat. 1998, 15, 21–26. [Google Scholar] [CrossRef]
- Adlard, P.A.; West, A.K.; Vickers, J.C. Increased density of metallothionein I/II-immunopositive cortical glial cells in the early stages of Alzheimer’s disease. Neurobiol. Dis. 1998, 5, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, J.; Penkowa, M.; Espejo, C.; Martínez-Cáceres, E.M.; Carrasco, J.; Quintana, A.; Molinero, A.; Florit, S.; Giralt, M.; Ortega-Aznar, A. Expression of metallothionein-I, -II, and -III in Alzheimer’s disease and animal models of neuroinflammation. Exp. Biol. Med. 2006, 231, 1450–1458. [Google Scholar] [CrossRef]
- Hidalgo, J.; Aschner, M.; Zatta, P.; Vašák, M. Roles of the metallothionein family of proteins in the central nervous system. Brain Res. Bull. 2001, 55, 133–145. [Google Scholar] [CrossRef]
- West, A.K.; Hidalgo, J.; Eddins, D.; Levin, E.D.; Aschner, M. Metallothionein in the central nervous system: Roles in protection, regeneration and cognition. Neurotoxicology 2008, 29, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Nam, Y.P.; Jeon, S.M.; Han, H.S.; Suk, K. Amyloid neurotoxicity is attenuated by metallothionein: Dual mechanisms at work. J. Neurochem. 2012, 121, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Manso, Y.; Carrasco, J.; Comes, G.; Adlard, P.A.; Bush, A.I.; Hidalgo, J. Characterization of the role of the antioxidant proteins metallothioneins 1 and 2 in an animal model of Alzheimer’s disease. Cell. Mol. Life Sci. 2012, 69, 3665–3681. [Google Scholar] [CrossRef] [PubMed]
- Manso, Y.; Comes, G.; López-Ramos, J.C.; Belfiore, M.; Molinero, A.; Giralt, M.; Carrasco, J.; Adlard, P.A.; Bush, A.I.; Delgado-García, J.M.; et al. Overexpression of metallothionein-1 modulates the phenotype of the Tg2576 mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 51, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Molinero, A.; Penkowa, M.; Hernandez, J.; Camats, J.; Giralt, M.; Lago, N.; Carrasco, J.; Campbell, I.; Hidalgo, J. Metallothionein-1 overexpression decreases brain pathology in transgenic mice with astrocyte-targeted expression of interleukin-6. J. Neuropathol. Exp. Neurol. 2003, 62, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Acarin, L.; González, B.; Hidalgo, J.; Castro, A.J.; Castellano, B. Primary cortical glial reaction versus secondary thalamic glial response in the excitotoxically injured young brain: Astroglial response and metallothionein expression. Neuroscience 1999, 92, 827–839. [Google Scholar] [CrossRef]
- Penkowa, M.; Carrasco, J.; Giralt, M.; Moos, T.; Hidalgo, J. CNS wound healing is severely depressed in metallothionein I and II-deficient mice. J. Neurosci. 1999, 19, 2535–2545. [Google Scholar] [PubMed]
- Chung, R.S.; Vickers, J.C.; Chuah, M.I.; West, A.K. Metallothionein-IIA promotes initial neurite elongation and postinjury reactive neurite growth and facilitates healing after focal cortical brain injury. J. Neurosci. 2003, 23, 3336–3342. [Google Scholar] [PubMed]
- Campbell, I.L.; Abraham, C.R.; Masliah, E.; Kemper, P.; Inglis, J.D.; Oldstone, M.B.A.; Mucke, L. Neurologic disease in transgenic mice by cerebral overexpression of interleukin 6. Proc. Natl. Acad. Sci. USA 1993, 90, 10061–10065. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-S.; Stalder, A.; Samimi, A.; Campbell, I.L. Reactive gliosis as a consequence of interleukin 6 expression in the brain: Studies in transgenic mice. Dev. Neurosci. 1994, 16, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; Adlard, P.; Cotman, C.; Quintana, A.; Penkowa, M.; Xu, F.; Van Nostrand, W.E.; Hidalgo, J. Metallothionein-I and -III expression in animal models of Alzheimer’s disease. Neuroscience 2006, 143, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Sandgren, E.P.; Koeller, D.M.; Brinster, R.L. Distal regulatory elements from the mouse metallothionein locus stimulate gene expression in transgenic mice. Mol. Cell. Biol. 1993, 13, 5266–5275. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.S.; Adlard, P.A.; Dittmann, J.; Vickers, J.C.; Chuah, M.I.; West, A.K. Neuron-glia communication: Metallothionein expression is specifically up-regulated by astrocytes in response to neuronal injury. J. Neurochem. 2004, 88, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.; Chapman, P.; Nilsen, S.; Eckman, C.; Harigaya, Y.; Younkin, S.; Yang, F.; Cole, G. Correlative memory deficits, abeta elevation, and amyloid plaques in transgenic mice. Science 1996, 274, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Kawarabayashi, T.; Younkin, L.H.; Saido, T.C.; Shoji, M.; Ashe, K.H.; Younkin, S.G. Age-dependent changes in brain, CSF, and plasma amyloid (β) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2001, 21, 372–381. [Google Scholar] [PubMed]
- Puttaparthi, K.; Gitomer, W.L.; Krishnan, U.; Son, M.; Rajendran, B.; Elliott, J.L. Disease progression in a transgenic model of familial amyotrophic lateral sclerosis is dependent on both neuronal and non-neuronal zinc binding proteins. J. Neurosci. 2002, 22, 8790–8796. [Google Scholar] [PubMed]
- Potter, E.G.; Cheng, Y.; Knight, J.B.; Gordish-Dressman, H.; Natale, J.E. Metallothionein I and II attenuate the thalamic microglial response following traumatic axotomy in the immature brain. J. Neurotrauma 2007, 24, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.S.; Leung, Y.K.; Butler, C.W.; Chen, Y.; Eaton, E.D.; Pankhurst, M.W.; West, A.K.; Guillemin, G.J. Metallothionein treatment attenuates microglial activation and expression of neurotoxic quinolinic acid following traumatic brain injury. Neurotox. Res. 2009, 15, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, M.E.; Wiederhold, K.H.; Abramowski, D.; Phinney, A.L.; Probst, A.; Sturchler-Pierrat, C.; Staufenbiel, M.; Sommer, B.; Jucker, M. Neuron loss in app transgenic mice. Nature 1998, 395, 755–756. [Google Scholar] [CrossRef] [PubMed]
- West, M.J.; Coleman, P.D.; Flood, D.G.; Troncoso, J.C. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 1994, 344, 769–772. [Google Scholar] [CrossRef]
- Asmussen, J.W.; Ambjørn, M.; Bock, E.; Berezin, V. Peptides modeled after the α-domain of metallothionein induce neurite outgrowth and promote survival of cerebellar granule neurons. Eur. J. Cell Biol. 2009, 88, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Penkowa, M.; Florit, S.; Giralt, M.; Quintana, A.; Molinero, A.; Carrasco, J.; Hidalgo, J. Metallothionein reduces central nervous system inflammation, neuro degeneration, and cell death following kainic acid-induced epileptic seizures. J. Neurosci. Res. 2005, 79, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Eidizadeh, A.; Khajehalichalehshtari, M.; Freyer, D.; Trendelenburg, G. Assessment of the therapeutic potential of metallothionein-ii application in focal cerebral ischemia in vitro and in vivo. PLoS ONE 2015, 10, e0144035. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I.; Moir, R.D.; Rosenkrantz, K.M.; Tanzi, R. Zinc and Alzheimer’s disease-response. Science 1995, 268, 1921–1923. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.J.; Cappai, R.; Volitakis, I.; Cherny, R.A.; White, A.R.; Beyreuther, K.; Masters, C.L.; Bush, A.I.; Li, Q.X. Overexpression of Alzheimer’s disease amyloid-β opposes the age-dependent elevations of brain copper and iron. J. Biol. Chem. 2002, 277, 44670–44676. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ruifrok, A.C.; Johnston, D.A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar] [PubMed]






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comes, G.; Manso, Y.; Escrig, A.; Fernandez-Gayol, O.; Sanchis, P.; Molinero, A.; Giralt, M.; Carrasco, J.; Hidalgo, J. Influence of Transgenic Metallothionein-1 on Gliosis, CA1 Neuronal Loss, and Brain Metal Levels of the Tg2576 Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 251. https://doi.org/10.3390/ijms18020251
Comes G, Manso Y, Escrig A, Fernandez-Gayol O, Sanchis P, Molinero A, Giralt M, Carrasco J, Hidalgo J. Influence of Transgenic Metallothionein-1 on Gliosis, CA1 Neuronal Loss, and Brain Metal Levels of the Tg2576 Mouse Model of Alzheimer’s Disease. International Journal of Molecular Sciences. 2017; 18(2):251. https://doi.org/10.3390/ijms18020251
Chicago/Turabian StyleComes, Gemma, Yasmina Manso, Anna Escrig, Olaya Fernandez-Gayol, Paula Sanchis, Amalia Molinero, Mercedes Giralt, Javier Carrasco, and Juan Hidalgo. 2017. "Influence of Transgenic Metallothionein-1 on Gliosis, CA1 Neuronal Loss, and Brain Metal Levels of the Tg2576 Mouse Model of Alzheimer’s Disease" International Journal of Molecular Sciences 18, no. 2: 251. https://doi.org/10.3390/ijms18020251
APA StyleComes, G., Manso, Y., Escrig, A., Fernandez-Gayol, O., Sanchis, P., Molinero, A., Giralt, M., Carrasco, J., & Hidalgo, J. (2017). Influence of Transgenic Metallothionein-1 on Gliosis, CA1 Neuronal Loss, and Brain Metal Levels of the Tg2576 Mouse Model of Alzheimer’s Disease. International Journal of Molecular Sciences, 18(2), 251. https://doi.org/10.3390/ijms18020251