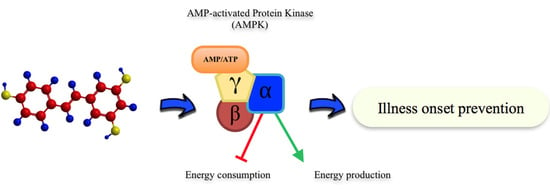
Adenosine Monophosphate (AMP)-Activated Protein Kinase: A New Target for Nutraceutical Compounds
Abstract
:1. Introduction
1.1. Mechanism of AMPK Activation
- Activation through the AMPK γ-subunit: Prodrugs that are converted into AMP analogs following cellular uptake by intracellular enzymes, such as 5-aminoimidazole-4-carboxamide riboside (AICAR). Upon entry the cell, AICAR is phosphorylated to generate Phosphorylated AICAriboside (ZMP), which works as an AMP mimetic. Another prodrug is compound 13 (C13), that generates isoxazole or C2, a potent activator and protector of α isoforms from dephosforylation.
- Direct activators binding between the β-CBM domain and kinase domain Ser108. Examples of these AMPK activating compounds are thienopyridone (A-769662), salicylate and 911 compound.
- A number of agents (metformin or hydrogen peroxide) and natural occurring products (berberin or resveratrol) act as mitochondria poisons (above mentioned) increasing the AMP:ATP ratio or energy charge and therefore activating AMPK indirectly.
1.2. Metabolic Functions and Physiological Regulation of AMPK
- The presence of 3′-4′-O-dihydroxy structure on the B aromatic ring (cathecol), which confers more stability and participates in electron delocalization. Studies have reported the relationship between cathecols and its involvement in the inhibition of lipid peroxidation [15].
- Hydroxyl groups in 5 and 7 positions on the A ring.
- Double bond localized in 2,3 positions conjugated with a 4-oxo group and the presence of 3-OH in C ring, responsible for the delocaization from B ring.
2. Nutraceutical Compounds and Cancer
3. Nutraceutical Compounds and Cardiovascular Disease
4. Nutraceutical Compounds and Type II Diabetes Mellitus
5. Nutraceutical Compounds and Neurodegenerative Diseases
6. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC1 | Acetyl-CoA carboxylase 1 |
| ACC2 | Acetyl-CoA carboxylase 2 |
| ADP | Adenosine diphosphate |
| AMP | Adenosine monophosphate |
| AMPK | Adenosine monophosphate-activated protein kinase |
| ATP | Adenosine triphosphate |
| CaMKKb | Ca2+/calmodulin-dependent protein kinase kinase-β |
| EGCG | Epillocatechin gallate |
| ERK | Extracellular signal-regulated kinases |
| GLUT4 | Glucose transporter type 4 |
| HbA1c | Glycated haemoglobin |
| HDAC | Histone deacetylase |
| HMG-CoA reductase | 3-hydroxy-3methyl-glutaryl-coenzyme A reductase |
| HOCl | Hypochlorous acid |
| HSV-1 | Herpex simplex virus 1 |
| HT | Hydroxytyrosol |
| ICAM-1 | Intracellular adhesion molecule 1 |
| iNOS | Inducible nitric oxide synthase |
| LKB1 | Liver kinase B1 |
| mTOR | Mammalian target of rapamycin |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| NF-κB | Nuclear Factor-κ β |
| PGC1α | Peroxisome proliferator-activated receptor gamma coactivator 1-α |
| PP2C | Protein phosphatase 2C |
| PRKAA1 | Protein Kinase AMP-Activated Catalytic Subunit α 1 |
| PRKAA2 | Protein Kinase AMP-Activated Catalytic Subunit α 2 |
| PRKAB1 | Protein Kinase AMP-Activated Non-Catalytic Subunit β 1 |
| PRKAB2 | Protein Kinase AMP-Activated Non-Catalytic Subunit β 2 |
| PRKAG1 | Protein Kinase AMP-Activated Non-Catalytic Subunit γ 1 |
| PRKAG2 | Protein Kinase AMP-Activated Non-Catalytic Subunit γ 2 |
| PRKAG3 | Protein Kinase AMP-Activated Non-Catalytic Subunit γ 3 |
| Raptor | Regulatory-associated protein of mTOR |
| SIRT1 | Sirtuin-1 |
| TBC1D1 | Tre-2/Bub2/Cdc16 domain family member |
| TIF-1A | Transcription initiation factor |
| TNF-α | Tumor necrosis factor α |
| TSC2 | Tuberous sclerosis complex 2 |
| ULK1 | Unc-51-like autophagy activating kinase 1 |
| ZMP | Phosphorylated AICAriboside |
References
- Cameron, K.O.; Kurumbail, R.G. Recent progress in the identification of adenosine monophosphate-activated protein kinase (AMPK) activators. Bioorg. Med. Chem. Lett. 2016, 26, 5139–5148. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, J.; Zhang, Y.-Y.; Yan, S.-F.; Newmann, D.; Schattner, U.; Wang, Z.-X.; Wu, J.-W. AMP-activated protein kinase undergoes nucleotide-dependent conformational changes. Nat. Struct. Mol. Biol. 2012, 19, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Alers, S.; Loffler, A.-S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Ducommun, S.; Deak, M.; Sumpton, D.; Ford, R.-J.; Nunez Galindo, A.; Kussmann, M.; Viollet, B.; Steinberg, G.-R.; Foretz, M.; Dayon, L.; et al. Motif affinity and mass spectrometry proteomic approach for the discovery of cellular AMPK targets: Identification of mitochondrial fission factor as a new AMPK substrate. Cell Signal. 2015, 27, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.-G. AMPK: Positive and negative regulation, and its role in whole-body energy homeostasis. Curr. Opin. Cell Biol. 2015, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Joungmok, K.; Goowon, Y.; Yeji, K.; Jin, K.; Joohun, H. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar]
- Miglianico, M.; Nicolaes, G.-A.; Neumann, D.-J. Pharmacological Targeting of AMP-Activated Protein Kinase and Opportunities for Computer-Aided Drug Design. J. Med. Chem. 2016, 59, 2879–2893. [Google Scholar] [CrossRef] [PubMed]
- Friedichsen, M.; Mortensen, B.; Pehmøller, C.; Birk, J.-B.; Wojtaszewski, J.-F. Exercise-induced AMPK activity in skeletal muscle: Role in glucose uptake and insulin sensitivity. Mol. Cell. Endocrinol. 2013, 366, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.-W.; Treeback, J.-T.; Wojtaszewski, J.-F.; Sakamoto, K. Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes 2011, 60, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Treebak, J.-T.; Pehmøller, C.; Kristensen, J.-M.; Kjøbsted, R.; Birk, J.-B.; Schjerling, P.; Richter, E.-A.; Goodyear, L.-J.; Wojtaszewski, J.-F. Acute exercise and physiological insulin induce distinct phosphorylation signatures on TBC1D1 and TBC1D4 proteins in human skeletal muscle. J. Physiol. 2014, 592, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-K.; Lee, W.S.; Kim, G.S.; Park, O.J. Anthocyanins are novel AMPKα1 stimulators that suppress tumor growth by inhibiting mTOR phosphorylation. Oncol. Rep. 2010, 24, 1471–1477. [Google Scholar] [PubMed]
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Hajiaghaalipour, F.; Khalilpourfarshbafi, M.; Arya, A. Modulation of glucose transporter protein by dietary flavonoids in type 2 diabetes mellitus. Int. J. Biol. Sci. 2015, 11, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Giampieri, F.; Alvarez Suarez, J.M.; Mazzoni, L.; Forbes Hernandez, T.Y.; Quiles, J.L.; Bullón, P.; Battino, M. AMPK as a New Attractive Therapeutic Target for Disease Prevention: The Role of Dietary Compounds AMPK and Disease Prevention. Curr. Drug Traget 2016, 17, 865–889. [Google Scholar] [CrossRef]
- Heim, K.-E.; Tagliaferro, A.-R.; Bobilya, D.-J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Lien, E.-J.; Ren, S.; Bui, H.-H.; Wang, R. Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic. Biol. Med. 1999, 26, 285–294. [Google Scholar] [CrossRef]
- Chao, J.; Leung, Y.; Wang, M.; Chang, R.C.-C. Nutraceuticals and their preventive or potential therapeutic value in Parkinson’s disease. Nutr. Rev. 2012, 70, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Park, S.Y.; Kim, Y.-M.; Lee, W.S.; Park, O.J. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp. Mol. Med. 2009, 41, 201. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.-P.I.; Tsai, S.-Y.; Kuo, Y.-H.; Pai, M.-H.; Chiu, H.-L.; Rodriguez, R.L.; Tang, F.-Y. Caffeic Acid Derivatives Inhibit the Growth of Colon Cancer: Involvement of the PI3-K/Akt and AMPK Signaling Pathways. PLoS ONE 2014, 9, e99631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Pasche, C.; Lucchini, F.; Ghidoni, R.; Ferraroni, M.; La Vecchia, C. Resveratrol and breast cancer risk. Eur. J. Cancer Prev. 2005, 14, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Hwang, J.-T.; Kwon, D.Y.; Surh, Y.-J.; Park, O.J. Induction of apoptosis by quercetin is mediated through AMPKα1/ASK1/p38 pathway. Cancer Lett. 2010, 292, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Park, O.J. Regulation of mutual inhibitory activities between AMPK and Akt with quercetin in MCF-7 breast cancer cells. Oncol. Rep. 2010, 24, 1493–1497. [Google Scholar] [PubMed]
- Kim, H.-J.; Kim, S.-K.; Kim, B.-S.; Lee, S.-H.; Park, Y.-S.; Park, B.-K.; Kim, S.-J.; Kim, J.; Choi, C.; Kim, J.-S.; et al. Apoptotic Effect of Quercetin on HT-29 Colon Cancer Cells via the AMPK Signaling Pathway. J. Agric. Food Chem. 2010, 58, 8643–8650. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-T.; Park, O.J.; Lee, Y.K.; Sung, M.J.; Hur, H.J.; Kim, M.S.; Ha, J.H.; Kwon, D.Y. Anti-tumor effect of luteolin is accompanied by AMP-activated protein kinase and nuclear factor-κB modulation in HepG2 hepatocarcinoma cells. Int. J. Mol. Med. 2011, 28, 25–31. [Google Scholar] [PubMed]
- Yang, J.-M.; Hung, C.-M.; Fu, C.-N.; Lee, J.-C.; Huang, C.-H.; Yang, M.-H.; Lin, C.-L.; Kao, J.-Y.; Way, T.-D. Hispidulin Sensitizes Human Ovarian Cancer Cells to TRAIL-Induced Apoptosis by AMPK Activation Leading to Mcl-1 Block in Translation. J. Agric. Food Chem. 2010, 58, 10020–10026. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-T.; Ha, J.; Park, I.-J.; Lee, S.K.; Baik, H.W.; Kim, Y.-M.; Park, O.J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007, 247, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-J.; Lee, Y.K.; Hwang, J.-T.; Kwon, D.Y.; Ha, J.; Park, O.J. Green Tea Catechin Controls Apoptosis in Colon Cancer Cells by Attenuation of H2O2-Stimulated COX-2 Expression via the AMPK Signaling Pathway at Low-Dose H2O2. Ann. N. Y. Acad. Sci. 2009, 1171, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-I.; Lee, Y.K.; Kim, Y.-M.; Hwang, J.-T.; Park, O.J. Possible link between NO concentrations and COX-2 expression in systems treated with soy-isoflavones. Ann. N. Y. Acad. Sci. 2007, 1095, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-T.; Ha, J.; Park, O.J. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem. Biophys. Res. Commun. 2005, 332, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-B.; Lee, M.S.; Cha, E.Y.; Lee, J.S.; Sul, J.Y.; Song, I.S.; Kim, J.Y. Magnolol-induced apoptosis in HCT-116 colon cancer cells is associated with the AMP-activated protein kinase signaling pathway. Biol. Pharm. Bull. 2012, 35, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.-S.; Park, K.-K. Magnolol suppresses metastasis via inhibition of invasion, migration, and matrix metalloproteinase-2/-9 activities in PC-3 human prostate carcinoma cells. Biosci. Biotechnol. Biochem. 2010, 74, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Szczepanski, M.-J.; Lee, Y.J. Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt signaling pathway in human prostate cancer cells. J. Cell. Biochem. 2009, 106, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Rehman, G.; Shehzad, A.; Khan, A.-L.; Hamayun, M. Role of AMP-activated protein kinase in cancer therapy. Arch. Pharm. 2014, 347, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.-R.; Sakamoto, K.; Bayascas, J.-R. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 2006, 75, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, A. The molecular basis and clinical aspects of Peutz-Jeghers syndrome. Cell. Mol. Life Sci. 1999, 55, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wullschleger, S.; Shpiro, N.; McGuire, V.-A.; Sakamoto, K.; Woods, Y.-L.; McBurnie, W.; Fleming, S.; Alessi, D.R. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem. J. 2008, 412, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.-M.; Donnelly, L.-A.; Emslie-Smith, A.-M.; Alessi, D.-R.; Morris, A.-D. Metformin and reduced risk of cancer in diabetic patients. Br. Med. J. 2005, 330, 1304–1305. [Google Scholar] [CrossRef] [PubMed]
- Budanov, A.-V.; Karin, M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 2008, 134, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, D.-M.; Shackelford, D.-B.; Egan, D.-F.; Mihaylova, M.-M.; Mery, A.; Vasquez, D.-S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, S.; Bierhoff, H.; Cado, I.; Weber, A.; Tiebe, M.; Grummt, I.; Voit, R. AMP-activated protein kinase adapts rRNA synthesis to cellular energy supply. Proc. Natl. Acad. Sci. USA 2009, 106, 17781–17786. [Google Scholar] [CrossRef] [PubMed]
- Laslett, L.J.; Alagona, P.; Clark, B.A.; Drozda, J.P.; Saldivar, F.; Wilson, S.R.; Poe, C.; Hart, M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012, 60, S1–S49. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, E250. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Kim, Y.-M.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Chang, K.C. Carbon monoxide (from CORM-2) inhibits high glucose-induced ICAM-1 expression via AMP-activated protein kinase and PPAR-gamma activations in endothelial cells. Atherosclerosis 2009, 207, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.S.; Wang, Z.B.; Ye, Z.; Lei, J.P.; Li, L.; Su, D.F.; Zheng, X. Resveratrol, an activator of SIRT1, upregulates AMPK and improves cardiac function in heart failure. Genet. Mol. Res. 2014, 13, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Soylemez, S.; Sepici, A.; Akar, F. Resveratrol Supplementation Gender Independently Improves Endothelial Reactivity and Suppresses Superoxide Production in Healthy Rats. Cardiovasc. Drugs Ther. 2009, 23, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Dyck, J.R.B. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim. Biophys. Acta. 2011, 1812, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, M.; Kawano, A.; Miura, Y.; Yagasaki, K. Hypoglycemic effect of resveratrol in type 2 diabetic model db/db mice and its actions in cultured L6 myotubes and RIN-5F pancreatic β-cells. J. Clin. Biochem. Nutr. 2011, 48, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Thandapilly, S.J.; Louis, X.L.; Yang, T.; Stringer, D.M.; Yu, L.; Zhang, S.; Wigle, J.; Kardami, E.; Zahradka, P.; Taylor, C.; et al. Resveratrol prevents norepinephrine induced hypertrophy in adult rat cardiomyocytes, by activating NO-AMPK pathway. Eur. J. Pharmacol. 2011, 668, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Z.; Reheman, A.; Jin, J.W.; Li, C.; Wang, Y.; Andrews, M.C.; Chen, P.; Zhu, G.; Ling, W.; et al. Plant Food Delphinidin-3-Glucoside Significantly Inhibits Platelet Activation and Thrombosis: Novel Protective Roles against Cardiovascular Diseases. PLoS ONE 2012, 7, e37323. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Kim, S.; Park, J.; Ha, T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2008, 373, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-T.; Kwon, D.Y.; Yoon, S.H. AMP-activated protein kinase: A potential target for the diseases prevention by natural occurring polyphenols. New Biotechnol. 2009, 26, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Suchankova, G.; Nelson, L.E.; Gerhart-Hines, Z.; Kelly, M.; Gauthier, M.-S.; Saha, A.K.; Ido, Y.; Puigserver, P.; Ruderman, N.B. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem. Biophys. Res. Commun. 2009, 378, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Croft, K.D.; Hodgson, J.M.; Kyle, R.; Lee, I.-L.E.; Wang, Y.; Stocker, R.; Ward, N.C. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem. Pharmacol. 2012, 84, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R. Hypochlorous Acid Impairs Endothelium-Derived Nitric Oxide Bioactivity through a Superoxide-Dependent Mechanism. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2028–2033. [Google Scholar] [CrossRef] [PubMed]
- Hazell, L.J.; Arnold, L.; Flowers, D.; Waeg, G.; Malle, E.; Stocker, R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J. Clin. Investig. 1996, 97, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK–FOXO3a pathway. Eur. J. Pharmacol. 2011, 660, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Porto, A.-G.; Brun, F.; Severini, G.-M.; Losurdo, P.; Fabris, E.; Taylor, M.-R.; Mestroni, L.; Sinagra, G. Clinical spectrum of PRKAG2 syndrome. Circ. Arrhythm. Electrophysiol. 2016, 9, e003121. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.K.; Doble, M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine 2009, 16, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Mohler, M.L.; He, Y.; Wu, Z.; Hwang, D.J.; Miller, D.D. Recent and emerging anti-diabetes targets. Med. Res. Rev. 2009, 29, 125–195. [Google Scholar] [CrossRef] [PubMed]
- Park, C.E.; Kim, M.-J.; Lee, J.H.; Min, B.-I.; Bae, H.; Choe, W.; Kim, S.-S.; Ha, J. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp. Mol. Med. 2007, 39, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Meerza, D.; Naseem, I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014, 109, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, S.; Ishida, S.; Hara, M.; Takahashi, N.; Yoshimatsu, H.; Sakata, T.; Korthuis, R.J. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radic. Biol. Med. 2003, 34, 810–817. [Google Scholar] [CrossRef]
- Van Ginkel, P.R.; Sareen, D.; Subramanian, L.; Walker, Q.; Darjatmoko, S.R.; Lindstrom, M.J.; Kulkarni, A.; Albert, D.M.; Polans, A.S. Resveratrol Inhibits Tumor Growth of Human Neuroblastoma and Mediates Apoptosis by Directly Targeting Mitochondria. Clin. Cancer Res. 2007, 13, 5162–5169. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, M.E.; Bertelli, A.E.; Fulgenzi, A.; Pellegatta, F.; Corsi, M.M.; Bonfrate, M.; Ferrara, F.; de Caterina, R.; Giovannini, L.; Bertelli, A. Activity in vitro of resveratrol on granulocyte and monocyte adhesion to endothelium. Am. J. Clin. Nutr. 1998, 68, 1208–1214. [Google Scholar] [PubMed]
- Puissant, A.; Auberger, P. AMPK- and p62/SQSTM1-dependent autophagy mediate Resveratrol-induced cell death in chronic myelogenous leukemia. Autophagy 2014, 6, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Um, J.-H.; Park, S.-J.; Kang, H.; Yang, S.; Foretz, M.; McBurney, M.W.; Kim, M.K.; Viollet, B.; Chung, J.H. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 2010, 59, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.M.; Sanli, T.; Giacca, A.; Tsiani, E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem. Biophys. Res. Commun. 2008, 374, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Do, G.-M.; Jung, U.J.; Park, H.-J.; Kwon, E.-Y.; Jeon, S.-M.; McGregor, R.A.; Choi, M.-S. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/dbmice. Mol. Nutr. Food Res. 2012, 56, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernández, T.-Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, C.; Yan, Y.; Chen, Q.; Luo, F.; Zhu, X.; Li, X.; Chen, K. Purification of naringin and neohesperidin from Huyou (Citrus changshanensis) fruit and their effects on glucose consumption in human HepG2 cells. Food Chem. 2012, 135, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Pu, P.; Gao, D.-M.; Mohamed, S.; Chen, J.; Zhang, J.; Zhou, X.-Y.; Zhou, N.-J.; Xie, J.; Jiang, H. Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch. Biochem. Biophys. 2012, 518, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, K.; Faubert, B.; MacNeil, J.; Tsiani, E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem. Biophys. Res. Commun. 2010, 398, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Tipoe, G.L.; Leung, T.-M.; Hung, M.-W.; Fung, M.-L. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc. Hematol. Disord. Drug Targets 2007, 7, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-H.; Chang, H.-H.; Lee, M.-J.; Chen, C.-L. Tea, obesity, and diabetes. Mol. Nutr. Food Res. 2006, 50, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Polansky, M.M. Tea Enhances Insulin Activity. J. Agric. Food Chem. 2002, 50, 7182–7186. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, S.; Wang, Y.; Thielecke, F. Anti-obesity effects of green tea: From bedside to bench. Mol. Nutr. Food Res. 2006, 50, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Klaus, S.; Pültz, S.; Thöne-Reineke, C.; Wolfram, S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. (Lond.) 2005, 29, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, Y. Minireview: Therapeutic potential of myricetin in diabetes mellitus. Food Sci. Hum. Wellness 2012, 1, 19–25. [Google Scholar] [CrossRef]
- Hardie, D.-G. AMP-activated protein kinase: Maintaining energy homeostasis at the cellular and whole-body levels. Annu. Rev. Nutr. 2014, 34, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.-G. AMPK: A target for drugs and natural products with effects on both diabetes and cancer. Diabetes 2013, 62, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Yamashita, H.; Hiemori, M.; Inagaki, E.; Kimoto, M.; Okamoto, M.; Tsuji, H.; Memon, A.N.; Mohammadio, A.; Natori, Y. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J. Nutr. Sci. Vitaminol. (Tokyo) 2007, 53, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Ka, E.H.; Lee, H.S.; Apostolidis, E.; Jang, H.D. Comparison of antioxidant potential and rat intestinal a-glucosidases inhibitory activities of quercetin, rutin, and isoquercetin. Int. J. Appl. Res. Nat. Prod. 2009, 2, 55–60. [Google Scholar]
- Kim, J.-H.; Kang, M.-J.; Choi, H.-N.; Jeong, S.-M.; Lee, Y.-M.; Kim, J.-I. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr. Res. Pract. 2011, 5, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Ding, Y.; Zhang, Z.; Cai, X.; Bao, L.; Li, Y. Quercetin but not quercitrin ameliorates tumor necrosis factor-alpha-induced insulin resistance in C2C12 skeletal muscle cells. Biol. Pharm. Bull. 2013, 36, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Jin, Y.C.; Chung, J.I.; Shin, S.C.; Lee, S.J.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. The anti-diabetic effect of anthocyanins in streptozotocin- induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol. Nutr. Food Res. 2009, 53, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 2006, 443, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Bredesen, D.E.; Rao, R.V.; Mehlen, P. Cell death in the nervous system. Nature 2006, 443, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Domise, M.; Vingtdeux, V. AMPK in Neurodegenerative Diseases. In AMP-Activated Protein Kinase; Springer: Cham, Switzerland, 2016; Volume 107, pp. 153–177. [Google Scholar]
- Tschäpe, J.-A.; Hammerschmied, C.; Mühlig-Versen, M.; Athenstaedt, K.; Daum, G.; Kretzschmar, D. The neurodegeneration mutant löchrig interferes with cholesterol homeostasis and Appl processing. EMBO J. 2002, 21, 6367–6376. [Google Scholar] [CrossRef] [PubMed]
- Spasic, M.R.; Callaerts, P.; Norga, K.K. Drosophila alicorn Is a Neuronal Maintenance Factor Protecting against Activity-Induced Retinal Degeneration. J. Neurosci. 2008, 28, 6419–6429. [Google Scholar] [CrossRef] [PubMed]
- Hang, L.; Basil, A.H.; Lim, K.-L. Nutraceuticals in Parkinson’s Disease. Neuromol. Med. 2016, 18, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Park, H.Y.; Kim, M.O. Anthocyanins Protect against Kainic Acid-induced Excitotoxicity and Apoptosis via ROS-activated AMPK Pathway in Hippocampal Neurons. CNS Neurosci. Ther. 2014, 20, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, D.; Zheng, Y.; Hu, B.; Zhang, Z.; Shan, Q.; Zheng, Z.; Liu, C.; Wang, Y. Quercetin activates AMP-activated protein kinase by reducing PP2C expression protecting old mouse brain against high cholesterol-induced neurotoxicity. J. Pathol. 2010, 222, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Tillu, D.V.; Melemedjian, O.K.; Asiedu, M.N.; Qu, N.; de Felice, M.; Dussor, G.; Price, T.J. Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain. Mol. Pain 2012, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Zhu, J.X.; Xie, W.; Le, W.; Fan, Z.; Jankovic, J.; Pan, T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals 2011, 19, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef] [PubMed]
- Leyton, L.; Hott, M.; Acuña, F.; Caroca, J.; Nuñez, M.; Martin, C.; Zambrano, A.; Concha, M.I.; Otth, C. Nutraceutical activators of AMPK/Sirt1 axis inhibit viral production and protect neurons from neurodegenerative events triggered during HSV-1 infection. Virus Res. 2015, 205, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Yan, L.; Jing, Y.Y.; Xu, L.H.; Liang, Y.D.; Wei, H.X.; Hu, B.; Pan, H.; Zha, Q.B.; Ouyang, D.Y.; et al. Berberine augments ATP-induced inflammasome activation in macrophages by enhancing AMPK signaling. Oncotarget 2016, 8, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Mei, F.; Wang, Y.; Xiao, N.; Yang, L.; Wang, Y.; Li, J.; Huang, F.; Kou, J.; Liu, B.; et al. Quercetin oppositely regulates insulin-mediated glucose disposal in skeletal muscle under normal and inflammatory conditions: The dual roles of AMPK activation. Mol. Nutr. Food Res. 2016, 60, 551–565. [Google Scholar] [CrossRef] [PubMed]





| NC | Classification | Pathway | Experimental Model | Comments | Nutrient | References |
|---|---|---|---|---|---|---|
 Luteolin | Flavone |
|
|
| Celery, parsley | [14] |
 EGCG | Flavonol |
|
|
| Fruits, vegetables, tea | [16,17] |
 Quercetin | Flavonol |
|
|
| Apple, grape, berries, onion, red wine, beans, broccoli, parsley | [12,13,19,22] |
 Genistein | Isoflavone |
|
|
| Legumes | [33] |
 Caffeic acid | Phenolic acid |
|
|
| Coffee, argan oil, thyme, sage, spearmint, ceylon cinnamon, star anise | [8] |
 Magnolol | Lignan |
|
|
| Roots and barks of species of Magnolia officinalis | [20] |
| NC | Classification | Pathway | Experimental Model | Comments | Nutrient | References |
|---|---|---|---|---|---|---|
 Hydroxytyrosol | Phenolic alcohol |
|
|
| Extra virgin olive oil, leaves from Olea Europea L. | [38] |
 Dp-3-glu | Anthocyanin |
|
|
| Bilberry fruits, cacao, pomegranate | [31] |
 Resveratrol | Stilbene |
|
|
| Skin of grapes, blueberries, raspberries, mulberries and red wine | [24] |
 Quercetin | Flavonol |
|
|
| Apple, grape, berries, onion, red wine, beans, broccoli, parsley | [35] |
| NC | Classification | Pathway | Experimental model | Comments | Nutrient | References |
|---|---|---|---|---|---|---|
 Naringin | Flavonona |
|
|
| Citrus fruits, some berries, tomatoes, mint | [4] |
 Naringenin | Flavonona |
|
|
| Citrus fruits, some berries, tomatoes, mint | [4] |
 Resveratrol | Stilbene |
|
|
| Skin of grapes, blueberries, raspberries, mulberries and red wine | [33] |
 Quercetin | Flavonol |
|
|
| Apple, grape, berries, onion, red wine, beans, broccoli, parsley | [59,60,61] |
 EGCG | Flavonol |
|
|
| Fruits, vegetables, tea | [57] |
 HT | Phenolic alcohol |
|
|
| Extra virgin olive oil, leaves from Olea Europea L. | [63] |
 Berberine | Alkaloids |
|
|
| Berberis spp. and other plants | [82,83] |
 Galegine | Alkaloids |
|
|
| Galega officinalis | [82,83] |
| NC | Classification | Pathway | Experimental model | Comments | Nutrient | References |
|---|---|---|---|---|---|---|
 Quercetin | Flavonol |
|
|
| Apple, berries, onion, red wine, beans, broccoli, parsley, green tea | [72] |
 Cyanidin | Anthocyanin |
|
|
| Red raspberries, soybean, peach, lychee, red oranges and rice | [71] |
 Resveratrol | Stilbene |
|
|
| Skin of grapes, blueberries, raspberries, mulberries and red wine | [74,76] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-Aguilar, F.; Pavillard, L.E.; Giampieri, F.; Bullón, P.; Cordero, M.D. Adenosine Monophosphate (AMP)-Activated Protein Kinase: A New Target for Nutraceutical Compounds. Int. J. Mol. Sci. 2017, 18, 288. https://doi.org/10.3390/ijms18020288
Marín-Aguilar F, Pavillard LE, Giampieri F, Bullón P, Cordero MD. Adenosine Monophosphate (AMP)-Activated Protein Kinase: A New Target for Nutraceutical Compounds. International Journal of Molecular Sciences. 2017; 18(2):288. https://doi.org/10.3390/ijms18020288
Chicago/Turabian StyleMarín-Aguilar, Fabiola, Luis E. Pavillard, Francesca Giampieri, Pedro Bullón, and Mario D. Cordero. 2017. "Adenosine Monophosphate (AMP)-Activated Protein Kinase: A New Target for Nutraceutical Compounds" International Journal of Molecular Sciences 18, no. 2: 288. https://doi.org/10.3390/ijms18020288
APA StyleMarín-Aguilar, F., Pavillard, L. E., Giampieri, F., Bullón, P., & Cordero, M. D. (2017). Adenosine Monophosphate (AMP)-Activated Protein Kinase: A New Target for Nutraceutical Compounds. International Journal of Molecular Sciences, 18(2), 288. https://doi.org/10.3390/ijms18020288