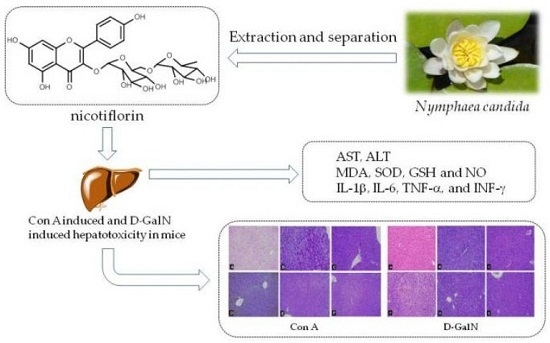
Hepatoprotective Effects of Nicotiflorin from Nymphaea candida against Concanavalin A-Induced and D-Galactosamine-Induced Liver Injury in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Protective Effect of Nicotiflorin on Con A Induced Hepatotoxicity in Mice
2.2. Protective Effect of Nicotiflorin on d-GalN-Induced Hepatotoxicity in Mice
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and Preparation of Nicotiflorin
3.3. Animals
3.4. Concanavalin A (Con A)-Induced Hepatotoxicity
3.5. d-Galactosamine (d-GalN)-Induced Hepatotoxicity
3.6. Measurement of Liver Index, Spleen Index and Body Weight in Mice
3.7. Measurement of Aminotransferase and Cytokine Levels in the Serum
3.8. Measurement of Liver Homogenate Contents of MDA, SOD, GSH and NO
3.9. Histopathological Examination
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghabril, M.; Chalasani, N.; Björnsson, E. Drug-induced liver injury: A clinical update. Curr. Opin. Gastroenterol. 2010, 26, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Quaranta, V.; Antonaci, S. Tissue remodelling in liver diseases. Histol. Histopathol. 2003, 18, 1267–1274. [Google Scholar] [PubMed]
- Upur, H.; Amat, N.; Blazekovic, B.; Talip, A. Protective effect of Cichorium glandulosum root extract on carbon tetrachloride-induced and galactosamine-induced hepatotoxicity in mice. Food Chem. Toxicol. 2009, 47, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, C.Y.; Lin, L.J.; Kao, S.T.; Lo, H.Y.; Chou, S.T.; Ho, T.Y. Glycyrrhizin, silymarin, and ursodeoxycholic acid regulate a common hepatoprotective pathway in HepG2 cells. Phytomedicine 2015, 22, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Q.; Lu, Y.F.; Pi, J.B. New insights into generalized hepatoprotective effects of oleanolic acid: Key roles of metallothionein and Nrf2 induction. Biochem. Pharm. 2008, 76, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Černý, D.; Lekić, N.; Váňová, K.; Muchová, L.; Hořínek, A.; Kmoníčková, E.; Zídek, Z.; Kameníková, L.; Farghali, H. Hepatoprotective effect of curcumin in lipopolysaccharide/galactosamine model of liver injury in rats: Relationship to HO-1/CO antioxidant system. Fitoterapia 2011, 82, 786–791. [Google Scholar]
- Tiegs, G.; Hentschel, J.; Wendel, A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Investig. 1992, 90, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, T.; Ma, L.; Yan, M.; Gu, Z.Y.; Huang, Y.; Xu, F.; Zhao, Y. Antioxidant and preventive effects of extract from Nymphaea candida flower on in vitro immunological liver injury of rat primary hepatocyte cultures. Evid. Complement. Altern. Med. 2009. [Google Scholar] [CrossRef]
- Tong, S.; Yan, J.; Chen, G.; Lou, J. Purification of rutin and nicotiflorin from the flowers of Edgeworthia chrysantha Lindl. by high speed counter-current chromatography. J. Chromatogr. Sci. 2009, 47, 341–344. [Google Scholar] [PubMed]
- Olajide, O.; Li, S.S.; Liu, H.T.; Chai, X.; Wang, Y.F.; Gao, X.M. Studies on chemical constituents and DPPH Free radical scavenging activity of Carthamus tinctorius L. Nat. Prod. Res. Dev. 2014, 26, 60–63. [Google Scholar]
- Zhao, J.; Yan, M.; He, J.H.; Huang, Y.; Zhao, Y. Flavonol glycosides from the flowers of Nymphaea Candida. Chin. JMAP 2008, 25, 115–117. [Google Scholar]
- Huang, J.L.; Fu, S.T.; Jiang, Y.Y.; Cao, Y.B.; Guo, M.L.; Wang, Y.; Xu, Z. Protective effects of nicotiflorin on reducing memory dysfunction, energy metabolism failure and oxidative stress in multi-infarct dementia model rats. Pharmcol. Biochem. Behav. 2007, 86, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Li, R.P.; Guo, M.L.; Zhang, G.; Xu, X.F.; Li, Q. Nicotiflorin reduces cerebral ischemic damage and upregulates endothelial nitric oxide synthase in primarily cultured ratcerebral blood vessel endothelial cells. J. Ethnopharmacol. 2006, 107, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Li, R.P.; Guo, M.L.; Zhang, G.; Xu, X.F.; Li, Q. Neuroprotection of nicotiflorin inpermanent focal cerebral ischemiaand in neuronal cultures. Biol. Pharm. Bull. 2006, 29, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. A-glucosidase inhibitory activity of kaempferol-3-O-rutinoside. Nat. Prod. Commun. 2011, 6, 201–203. [Google Scholar] [PubMed]
- Wang, Y.; Tang, C.Y.; Zhang, H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. J. Food Drug Anal. 2015, 23, 310–317. [Google Scholar] [CrossRef]
- Imose, M.; Nagaki, M.; Kimura, K.; Takai, S.; Imao, M.; Naiki, T. Leflunomide protects from T-cell-mediated liver injury in mice through inhibition of nuclear factor κB. Hepatology 2004, 40, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhu, H.H.; Zhou, L.F.; Li, J.; Zhao, L.Y.; Wu, S.S.; Wang, J.; Liu, W.; Chen, Z. Genes related to the very early stage of Con A-induced fulminant hepatitis: A gene-chip-based study in a mouse model. BMC Genom. 2010, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, K.; He, L.; Xia, Y.; Dai, W.; Wang, F.; Li, J.; Li, S.; Liu, T.; Zheng, Y.; et al. The protective effect of resveratrol on concanavalin-A induced acute hepatic injury in mice. Gastroenterol. Res. Pract. 2015, 2015, 506390. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.; Heinlein, S.; Agli, A.; Bang, R.; Schumann, J.; Tiegs, G. Cytokine expression in three mouse models of experimental hepatitis. Cytokine 2002, 19, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qin, X.Y.; Guo, Z.; Liu, T.Y.; Pan, R.Y. Protective effect of ginsenoside Rg1 on immue-mediated liver injury in mice. Chin. J. Public Health 2015, 31, 309–311. [Google Scholar]
- Pan, C.W.; Zhou, G.Y.; Chen, W.L.; Lu, Z.G.; Jin, L.X.; Zheng, Y.; Lin, W.; Pan, Z.Z. Protective effect of forsythiaside A on lipopolysaccharide/d-galactosamine-induced liver injury. Int. Immunopharmacol. 2015, 26, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Ksontini, R.; Colagiovanni, D.B.; Josephs, M.D.; Edwards, C.K.; Tannahill, C.L.; Solorzano, U. Disparate roles for TNF-α and fasligand in concanavalin A-induced hepatitis. J. Immunol. 1998, 160, 4082–4089. [Google Scholar] [PubMed]
- Kimikide, N.; Mitsuyoshi, O.; Masashi, Y.; Shujiro, T.; Yukiomi, N.; Keisuke, T.; Kazunobu, A.; Isao, M. Macrophage inflammatory protein-2 induced by TNF-α plays a pivotal role in concanavalin A-induced liver injury in mice. J. Hepatol. 2001, 35, 217–224. [Google Scholar]
- Sinha, M.; Manna, P.; Sil, P.C. Amelioration of galactosamine-induced nephrotoxicity by a protein isolated from the leaves of the herb, Cajanus indicus L. BMC Complement. Altern. Med. 2007, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Ijiri, M.; Suzuki, T.; Taguchi, K.; Koyama, Y.; Isemura, M. Green tea with a high catechin content suppresses inflammatory cytokine expression in the galactosamine-injured rat liver. Biomed. Res. 2005, 26, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kang, J.W.; Kim, D.W.; Sung, Y.K.; Lee, S.M. Protective effects of Mg-CUD against d-galactosamine-induced hepatotoxicity in rats. Eur. J. Pharm. 2011, 657, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Huang, Z.M.; Yang, X.B.; Chen, H.Y. Protective effect of hyperin on immunological liver injury in mice. Chin. J. Exp. Tradit. Med. Fomulae 2015, 21, 137–141. [Google Scholar]
- Ai, G.; Huang, Z.M.; Liu, Q.C.; Han, Y.Q.; Chen, X. The protective effect of total phenolics from Oenanthe Javanica on acute liver failure induced by d-galactosamine. J. Ethnopharmacol. 2016, 186, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Wang, J.Q.; Xia, L.J.; Jiang, H.; Jing, D. The protective effects of total flavonoids Chrysanthemum indicum on CCl4-induced acute liver injury in mice. Acta Univ. Med. Anhui 2007, 42, 412–414. [Google Scholar]









| Group | Inital BW (g) | Final BW (g) | Liver Index | Speen Index |
|---|---|---|---|---|
| Control | 19.83 ± 0.37 | 26.49 ± 0.39 | 48.12 ± 0.80 | 3.65 ± 0.18 |
| Con A | 20.41 ± 0.49 | 27.44 ± 0.65 | 79.59 ± 2.20 ## | 7.19 ± 1.42 ## |
| DDB (150 mg/kg bw) + Con A | 20.01 ± 0.53 | 26.53 ± 0.57 | 55.82 ± 0.63 ** | 5.01 ± 0.22 ** |
| nicotiflorin (25 mg/kg bw) + Con A | 19.84 ± 0.47 | 26.64 ± 0.40 | 61.82 ± 1.22 ** | 5.48 ± 0.27 ** |
| nicotiflorin (50 mg/kg bw) + Con A | 20.11 ± 0.70 | 27.09 ± 0.41 | 57.39 ± 2.15 ** | 4.89 ± 0.36 ** |
| nicotiflorin (100 mg/kg bw) + Con A | 21.69 ± 0.31 | 28.18 ± 0.37 | 53.98 ± 1.13 ** | 4.24 ± 0.20 ** |
| Group | 0 | I | II | III | p-Value |
|---|---|---|---|---|---|
| Control | 10 | 0 | 0 | 0 | - |
| Con A | 0 | 0 | 2 | 8 | # |
| DDB (150 mg/kg bw) + Con A | 1 | 5 | 2 | 2 | * |
| nicotiflorin (25 mg/kg bw) + Con A | 2 | 6 | 1 | 1 | * |
| nicotiflorin (50 mg/kg bw) + Con A | 2 | 5 | 2 | 1 | * |
| nicotiflorin (100 mg/kg bw) + Con A | 2 | 6 | 1 | 1 | * |
| Group | Inital BW (g) | Final BW (g) | Liver Index | Speen Index |
|---|---|---|---|---|
| Control | 20.72 ± 0.59 | 25.02 ± 1.11 | 45.12 ± 1.13 | 4.25 ± 0.22 |
| d-GalN | 20.28 ± 0.34 | 26.35 ± 0.72 | 84.44 ± 1.47 ## | 8.26 ± 0.20 ## |
| DDB (150 mg/kg bw) + d-GalN | 21.36 ± 0.42 | 26.38 ± 0.58 | 53.75 ± 1.09 ** | 5.54 ± 0.18 ** |
| nicotiflorin (25 mg/kg bw) + d-GalN | 21.25 ± 0.48 | 25.64 ± 0.46 | 65.44 ± 0.89 ** | 6.43 ± 0.57 ** |
| nicotiflorin (50 mg/kg bw) + d-GalN | 21.26 ± 0.59 | 26.13 ± 0.80 | 57.48 ± 2.46 ** | 5.55 ± 0.30 ** |
| nicotiflorin (100 mg/kg bw) + d-GalN | 20.71 ± 0.59 | 26.75 ± 0.56 | 49.35 ± 1.50 ** | 4.88 ± 0.23 ** |
| Group | 0 | I | II | III | p-Value |
|---|---|---|---|---|---|
| Control | 10 | 0 | 0 | 0 | - |
| d-GalN | 0 | 0 | 3 | 7 | # |
| DDB (150 mg/kg bw) + d-GalN | 1 | 6 | 2 | 1 | * |
| nicotiflorin (25 mg/kg bw) + d-GalN | 1 | 5 | 3 | 1 | * |
| nicotiflorin (50 mg/kg bw) + d-GalN | 2 | 4 | 3 | 1 | * |
| nicotiflorin (100 mg/kg bw) + d-GalN | 3 | 5 | 1 | 1 | * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Zhang, S.; You, S.; Liu, T.; Xu, F.; Ji, T.; Gu, Z. Hepatoprotective Effects of Nicotiflorin from Nymphaea candida against Concanavalin A-Induced and D-Galactosamine-Induced Liver Injury in Mice. Int. J. Mol. Sci. 2017, 18, 587. https://doi.org/10.3390/ijms18030587
Zhao J, Zhang S, You S, Liu T, Xu F, Ji T, Gu Z. Hepatoprotective Effects of Nicotiflorin from Nymphaea candida against Concanavalin A-Induced and D-Galactosamine-Induced Liver Injury in Mice. International Journal of Molecular Sciences. 2017; 18(3):587. https://doi.org/10.3390/ijms18030587
Chicago/Turabian StyleZhao, Jun, Shilei Zhang, Shuping You, Tao Liu, Fang Xu, Tengfei Ji, and Zhengyi Gu. 2017. "Hepatoprotective Effects of Nicotiflorin from Nymphaea candida against Concanavalin A-Induced and D-Galactosamine-Induced Liver Injury in Mice" International Journal of Molecular Sciences 18, no. 3: 587. https://doi.org/10.3390/ijms18030587
APA StyleZhao, J., Zhang, S., You, S., Liu, T., Xu, F., Ji, T., & Gu, Z. (2017). Hepatoprotective Effects of Nicotiflorin from Nymphaea candida against Concanavalin A-Induced and D-Galactosamine-Induced Liver Injury in Mice. International Journal of Molecular Sciences, 18(3), 587. https://doi.org/10.3390/ijms18030587