Phosphorus and Iron Deficiencies Influences Rice Shoot Growth in an Oxygen Dependent Manner: Insight from Upland and Lowland Rice
Abstract
:1. Introduction
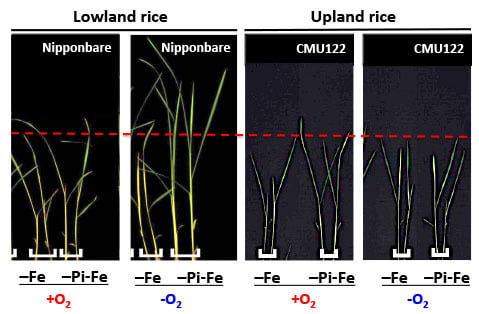
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rehman, H.; Aziz, T.; Farooq, M.; Wakeel, A.; Rengel, Z. Zinc nutrition in rice production systems: A review. Plant Soil 2012, 361, 203–226. [Google Scholar] [CrossRef]
- Sun, F.; Kolvenbach, B.A.; Nastold, P.; Jiang, B.; Ji, R.; Corvini, P.F.X. Degradation and metabolism of Tetrabromobisphenol A (TBBPA) in submerged soil and soil-plant systems. Environ. Sci. Technol. 2014, 48, 14291–14299. [Google Scholar] [PubMed]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Serraj, R.; McNally, K.L.; Slamet-Loedin, I.; Kohli, A.; Haefele, S.M.; Atlin, G.; Kumar, A. Drought resistance improvement in rice: An integrated genetic and resource management strategy. Plant Prod. Sci. 2011, 14, 1–14. [Google Scholar] [CrossRef]
- Fukao, T.; Xiong, L. Genetic mechanisms conferring adaptation to submergence and drought in rice: Simple or complex? Curr. Opin. Plant Biol. 2013, 16, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Mongon, J.; Konnerup, D.; Colmer, T.D.; Rerkasem, B. Responses of rice to Fe2+ in aerated and stagnant conditions: Growth, root porosity and radial O2 loss barrier. Funct. Plant Biol. 2014, 41, 922–929. [Google Scholar] [CrossRef]
- Atwell, B.J.; Greenway, H.; Colmer, T.D. Efficient use of energy in anoxia-tolerant plants with focus on germinating rice seedlings. New Phytol. 2015, 206, 36–56. [Google Scholar] [CrossRef] [PubMed]
- Colmer, T.D.; Cox, M.C.H.; Voesenek, L.A.C.J. Root aeration in rice (Oryza sativa): Evaluation of O2, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytol. 2006, 170, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flooding tolerance: O2 sensing and survival strategies. Curr. Opin. Plant Biol. 2013, 16, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Colmer, T.D. Aerenchyma and an inducible barrier to radial O2 loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann. Bot. 2003, 91, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Secco, D.; Bouain, N.; Rouached, A.; Prom-u-thai, C.; Hanin, M.; Pandey, A.K.; Rouached, H. Phosphate, phytate and phytases in plants: From fundamental knowledge gained in Arabidopsis to potential biotechnological applications in wheat. Crit. Rev. Biotechnol. 2017, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; Lopez-Arredondo, D.; Wissuwa, M.; Delhaize, E.; Rouached, H. Improving phosphorus use efficiency—A complex trait with emerging opportunities. Plant J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.; Rouached, H.; Tissot, N.; Gaymard, F.; Dubos, C. Integration of P, S, Fe and Zn nutrition signals in Arabidopsis thaliana: Potential involvement of PHOSPHATE STARVATION RESPONSE1 (PHR1). Front. Plant Sci. 2015, 6, 290. [Google Scholar] [CrossRef] [PubMed]
- Saenchai, C.; Bouain, N.; Kisko, M.; Prom-u-thai, C.; Doumas, P.; Rouached, H. The involvement of OsPHO1;1 in the regulation of iron transport through integration of phosphate and zinc deficiency signaling. Front. Plant Sci. 2016, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Huang, F.; Narsai, R.; Wu, J.; Giraud, E.; He, F.; Cheng, L.; Wang, F.; Wu, P.; Whelan, J.; et al. Physiological and transcriptome analysis of iron and phosphorus interaction in rice seedlings. Plant Physiol. 2009, 151, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Bouain, N.; Kisko, M.; Rouached, A.; Dauzat, M.; Lacombe, B.; Belgaroui, N.; Ghnaya, T.; Davidian, J.C.; Berthomieu, P.; Abdelly, C.; et al. Phosphate/zinc interaction analysis in two lettuce varieties reveals contrasting effects on biomass, photosynthesis, and dynamics of Pi transport. BioMed Res. Int. 2014, 2014, 548254. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, Z.; Canut, M.; Tournaire-Roux, C.; Martinière, A.; Boursiac, Y.; Loudet, O.; Maurel, C. A potassium-dependent oxygen sensing pathway regulates plant root hydraulics. Cell 2016, 167, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Rouached, H.; Secco, D.; Arpat, B.A. Regulation of ion homeostasis in plants: Current approaches and future challenges. Plant Signal. Behav. 2010, 5, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Foorno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice, 3rd ed.; International Rice Research Institute: Los Baños, Philippines, 1976; pp. 61–63. [Google Scholar]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongon, J.; Chaiwong, N.; Bouain, N.; Prom-u-thai, C.; Secco, D.; Rouached, H. Phosphorus and Iron Deficiencies Influences Rice Shoot Growth in an Oxygen Dependent Manner: Insight from Upland and Lowland Rice. Int. J. Mol. Sci. 2017, 18, 607. https://doi.org/10.3390/ijms18030607
Mongon J, Chaiwong N, Bouain N, Prom-u-thai C, Secco D, Rouached H. Phosphorus and Iron Deficiencies Influences Rice Shoot Growth in an Oxygen Dependent Manner: Insight from Upland and Lowland Rice. International Journal of Molecular Sciences. 2017; 18(3):607. https://doi.org/10.3390/ijms18030607
Chicago/Turabian StyleMongon, Jenjira, Nanthana Chaiwong, Nadia Bouain, Chanakan Prom-u-thai, David Secco, and Hatem Rouached. 2017. "Phosphorus and Iron Deficiencies Influences Rice Shoot Growth in an Oxygen Dependent Manner: Insight from Upland and Lowland Rice" International Journal of Molecular Sciences 18, no. 3: 607. https://doi.org/10.3390/ijms18030607
APA StyleMongon, J., Chaiwong, N., Bouain, N., Prom-u-thai, C., Secco, D., & Rouached, H. (2017). Phosphorus and Iron Deficiencies Influences Rice Shoot Growth in an Oxygen Dependent Manner: Insight from Upland and Lowland Rice. International Journal of Molecular Sciences, 18(3), 607. https://doi.org/10.3390/ijms18030607