On the Edge of Research and Technological Application: A Critical Review of Electromethanogenesis
Abstract
:1. Introduction
1.1. Bioelectrochemical Methane Production: Etymology
1.2. Bioelectrochemical Methane Production: Timeline
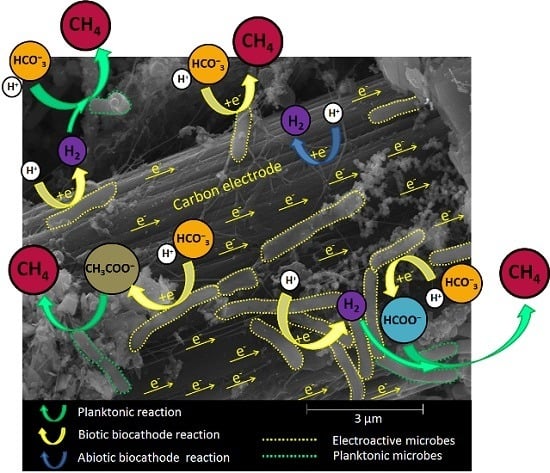
2. Electromethanogenesis Pathways, Microbial Communities and Proposed Functionalities
Electron Transfer from Cathode to Microbes in Electromethanogenesis
3. Applications of Electromethanogenesis
3.1. Renewable Energy Storage: The Bioelectrochemical Power-to-Methane Concept
3.2. Electromethanogenesis for Biogas Upgrading
3.3. Electromethanogenesis Coupled to Waste Treatment
4. Current Limitations in Electromethanogenesis and Proposed Strategies
4.1. Side/Parasitic Reactions
4.2. Mass Transport
4.3. Inoculum Type
4.4. Electrode
4.5. Anode-Cathode Separation: Membrane
4.6. Operation Parameters
4.7. System Design and Scaling-Up
5. Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestion |
| BES | Bioelectrochemical system |
| CCE | Cathode capture efficiency |
| CE | Coulombic efficiency |
| COD | Chemical oxygen demand |
| IET | Interspecies electron transfer |
| GF | Graphite felt |
| SHE | Standard hydrogen electrode |
| WWT | Wastewater treatment |
References
- Kotelnikova, S. Microbial production and oxidation of methane in deep subsurface. Earth Sci. Rev. 2002, 58, 367–395. [Google Scholar] [CrossRef]
- Thauer, R.K.; Jungermann, K.; Decker, K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 1977, 41, 100–180. [Google Scholar] [PubMed]
- Rostrup-Nielsen, J.R.; Pedersen, K.; Sehested, J. High temperature methanation: Sintering and structure sensitivity. Appl. Catal. A Gen. 2007, 330, 134–138. [Google Scholar] [CrossRef]
- Sabatier, P.; Senderens, J.B. New synthesis of methane. C. R. Chim. 1902, 134, 514–516. [Google Scholar]
- Ryi, S.-K.; Lee, S.-W.; Hwang, K.-R.; Park, J.-S. Production of synthetic natural gas by means of a catalytic nickel membrane. Fuel 2012, 94, 64–69. [Google Scholar] [CrossRef]
- Khorsand, K.; Marvast, M.A.; Pooladian, N.; Kakavand, M. Modeling and simulation of methanation catalytic reactor in Ammonia unit. Pet. Coal 2007, 49, 46–53. [Google Scholar]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kawaguchi, H.; Kobayashi, H. Bio-electrochemical conversion of carbon dioxide to methane in geological storage reservoirs. Energy Convers. Manag. 2013, 66, 343–350. [Google Scholar] [CrossRef]
- Zhen, G.; Kobayashi, T.; Lu, X.; Xu, K. Understanding methane bioelectrosynthesis from carbon dioxide in a two-chamber microbial electrolysis cells (MECs) containing a carbon biocathode. Bioresour. Technol. 2015, 186, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Siegert, M.; Yates, M.D.; Call, D.F.; Zhu, X.; Spormann, A.M.; Logan, B.E. Comparison of nonprecious metal cathode materials for methane production by electromethanogenesis. ACS Sustain. Chem. Eng. 2014, 2, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Saito, N.; Fu, Q.; Kawaguchi, H.; Vilcaez, J.; Wakayama, T.; Maeda, H.; Sato, K. Bio-electrochemical property and phylogenetic diversity of microbial communities associated with bioelectrodes of an electromethanogenic reactor. J. Biosci. Bioeng. 2013, 116, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef] [PubMed]
- Batlle-Vilanova, P.; Puig, S.; Gonzalez-Olmos, R.; Vilajeliu-Pons, A.; Balaguer, M.D.; Colprim, J. Deciphering the electron transfer mechanisms for biogas upgrading to biomethane within a mixed culture biocathode. RSC Adv. 2015, 5, 52243–52251. [Google Scholar] [CrossRef]
- Fu, Q.; Kuramochi, Y.; Fukushima, N.; Maeda, H.; Sato, K.; Kobayashi, H. Bioelectrochemical analyses of the development of a thermophilic biocathode catalyzing electromethanogenesis. Environ. Sci. Technol. 2015, 49, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Aulenta, F.; Villano, M.; Angenent, L.T. Cathodes as electron donors for microbial metabolism: Which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 2011, 102, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Van Eerten-Jansen, M.C.A.A.; Jansen, N.C.; Plugge, C.M.; de Wilde, V.; Buisman, C.J.; Ter Heijne, A. Analysis of the mechanisms of bioelectrochemical methane production by mixed cultures. J. Chem. Technol. Biotechnol. 2015, 90, 963–970. [Google Scholar] [CrossRef]
- Van Eerten-Jansen, M.C.A.A.; Ter Heijne, A.; Buisman, C.J.; Hamelers, H.V.M. Microbial electrolysis cells for production of methane from CO2: Long-term performance and perspectives. Int. J. Energy Res. 2012, 31, 135–147. [Google Scholar] [CrossRef]
- Van Eerten-Jansen, M.C.A.A.; Veldhoen, A.B.; Plugge, C.M.; Stams, A.J.M.; Buisman, C.J.; Ter Heijne, A. Microbial community analysis of a methane-producing biocathode in a bioelectrochemical system. Archaea 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Lu, X.; Kobayashi, T.; Kumar, G.; Xu, K. Promoted electromethanosynthesis in a two-chamber microbial electrolysis cells (MECs) containing a hybrid biocathode covered with graphite felt (GF). Chem. Eng. J. 2016, 284, 1146–1155. [Google Scholar] [CrossRef]
- Marshall, C.; LaBelle, T.; Fichot, E.B.; Ross, D.E.; Norman, R.; May, H.D. Electroautotrophic Synthesis of Acetate and Methane. In Proceedings of the American Institute of Chemical Engineers 2012 Annual Meeting, Pittsburgh, PA, USA, 28 October–2 November 2012. [Google Scholar]
- Geppert, F.; Liu, D.; van Eerten-Jansen, M.; Weidner, E.; Buisman, C.; Ter Heijne, A. Bioelectrochemical power-to-gas: State of the art and future perspectives. Trends Biotechnol. 2016, 34, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Schröder, U.; Harnisch, F.; Angenent, L.T. Microbial electrochemistry and technology: Terminology and classification. Energy Environ. Sci. 2015, 8, 513–519. [Google Scholar] [CrossRef]
- Schröder, U. Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys. Chem. Chem. Phys. 2007, 9, 2619–2629. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.; Belay, N.; Rajagopal, B.S.; Weimer, P.J. Bacterial methanogenesis and growth from CO2 with elemental iron as the sole source of electrons. Science 1987, 237, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Laivenieks, M.; Guettler, M.V.; Jain, M.K.; Zeikus, J.G. Microbial utilization of electrically reduced neutral red as the sole electron donor for growth and metabolite production. Appl. Environ. Microbiol. 1999, 65, 2912–2917. [Google Scholar] [PubMed]
- Clauwaert, P.; Tolêdo, R.; van der Ha, D.; Crab, R.; Verstraete, W.; Hu, H.; Udert, K.M.; Rabaey, K. Combining biocatalyzed electrolysis with anaerobic digestion. Water Sci. Technol. 2008, 57, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Jeremiasse, A.W.; Hamelers, H.V.M.; Buisman, C.J. Hydrogen production with a microbial biocathode. Environ. Sci. Technol. 2008, 42, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B. Electromethanogenic Reactor and Processes for Methane Production. U.S. Patent 20,090,317,882 A1, 24 December 2009. [Google Scholar]
- Morita, M.; Malvankar, N.S.; Franks, A.E.; Summers, Z.M.; Giloteaux, L.; Rotaru, A.-E.; Rotaru, C.; Lovley, D.R. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. MBio 2011, 2, e00159-11. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, B.; Mehta, P.; Bourque, J.-S.; Guiot, S.R. Electrolysis-enhanced anaerobic digestion of wastewater. Bioresour. Technol. 2011, 102, 5685–5691. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, K.; Holmes, D.E. Bioelectrochemical removal of carbon dioxide (CO2): An innovative method for biogas upgrading. Bioresour. Technol. 2014, 173, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef] [PubMed]
- Batlle-Vilanova, P.; Puig, S.; Gonzalez-Olmos, R.; Vilajeliu-Pons, A.; Bañeras, L.; Balaguer, M.D.; Colprim, J. Assessment of biotic and abiotic graphite cathodes for hydrogen production in microbial electrolysis cells. Int. J. Hydrog. Energy 2014, 39, 1297–1305. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Bretschger, O.; Carpenter, K.; Phan, T.; Suzuki, S.; Ishii, S.; Grossi-Soyster, E.; Flynn, M.; Hogan, J. Functional and taxonomic dynamics of an electricity-consuming methane-producing microbial community. Bioresour. Technol. 2015, 195, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.; Ross, D.; Handley, K.; Weisenhorn, P.; Edirisinghe, J.; Henry, C.; Gilbert, J.; May, H.; Norman, R.S. Metabolic reconstruction and modeling microbial electrosynthesis. BioRxiv 2016. [Google Scholar] [CrossRef]
- Lovley, D.R. Powering microbes with electricity: Direct electron transfer from electrodes to microbes. Environ. Microbiol. Rep. 2011, 3, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Geelhoed, J.S.; Hamelers, H.V.; Stams, A.J. Electricity-mediated biological hydrogen production. Curr. Opin. Microbiol. 2010, 13, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.W.; Ross, D.E.; Fichot, E.B.; Norman, R.S.; May, H.D. Long-term operation of microbial electrosynthesis systems improves acetate production by autotrophic microbiomes. Environ. Sci. Technol. 2013, 47, 6023–6029. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Morita, M.; Sasaki, D.; Hirano, S.; Matsumoto, N.; Ohmura, N.; Igarashi, Y. Methanogenic communities on the electrodes of bioelectrochemical reactors without membranes. J. Biosci. Bioeng. 2011, 111, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Su, M.; Li, D. Removal of sulfide and production of methane from carbon dioxide in microbial fuel cells–microbial electrolysis cell (MFCs–MEC) coupled system. Appl. Biochem. Biotechnol. 2014, 172, 2720–2731. [Google Scholar] [CrossRef] [PubMed]
- Siegert, M.; Yates, M.D.; Spormann, A.M.; Logan, B.E. Methanobacterium dominates biocathodic archaeal communities in methanogenic microbial electrolysis cells. ACS Sustain. Chem. Eng. 2015, 3, 1668–1676. [Google Scholar] [CrossRef]
- Xafenias, N.; Mapelli, V. Performance and bacterial enrichment of bioelectrochemical systems during methane and acetate production. Int. J. Hydrog. Energy 2014, 39, 21864–21875. [Google Scholar] [CrossRef]
- Deutzmann, J.S.; Spormann, A.M. Enhanced microbial electrosynthesis by using defined co-cultures. ISME J. 2017, 11, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Lohner, S.T.; Deutzmann, J.S.; Logan, B.E.; Leigh, J.; Spormann, A.M. Hydrogenase-independent uptake and metabolism of electrons by the archaeon Methanococcus maripaludis. ISME J. 2014, 8, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Ito, K.; Mori, K.; Tsurumaru, H.; Harayama, S. Iron-corroding methanogen isolated from a crude-oil storage tank. Appl. Environ. Microbiol. 2010, 76, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Deutzmann, J.S.; Sahin, M.; Spormann, A.M. Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. MBio 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dinh, H.T.; Kuever, J.; Mussmann, M.; Hassel, A.W.; Stratmann, M.; Widdel, F. Iron corrosion by novel anaerobic microorganisms. Nature 2004, 427, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Beese-Vasbender, P.F.; Grote, J.P.; Garrelfs, J.; Stratmann, M.; Mayrhofer, K.J.J. Selective microbial electrosynthesis of methane by a pure culture of a marine lithoautotrophic archaeon. Bioelectrochemistry 2015, 102, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Onaka, Y.; Kobayashi, H.; Fu, Q.; Kawaguchi, H.; Vilcaez, J.; Sato, K. Mechanism of electromethanogenic reduction of CO2 by a thermophilic methanogen. Energy Procedia 2013, 37, 7021–7028. [Google Scholar] [CrossRef]
- Sasaki, K.; Hirano, S.I.; Morita, M.; Sasaki, D.; Matsumoto, N.; Ohmura, N.; Igarashi, Y. Bioelectrochemical system accelerates microbial growth and degradation of filter paper. Appl. Microbiol. Biotechnol. 2011, 89, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.W.; Ross, D.E.; Fichot, E.B.; Norman, R.S.; May, H.D. Electrosynthesis of commodity chemicals by an autotrophic microbial community. Appl. Environ. Microbiol. 2012, 78, 8412–8420. [Google Scholar] [CrossRef] [PubMed]
- Zeppilli, M.; Villano, M.; Aulenta, F.; Lampis, S.; Vallini, G.; Majone, M. Effect of the anode feeding composition on the performance of a continuous-flow methane-producing microbial electrolysis cell. Environ. Sci. Pollut. Res. Int. 2015, 22, 7349–7360. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, H.; Takagi, K.; Fujita, M.; Kano, K.; Ikeda, T. Electrochemical study of reversible hydrogenase reaction of Desulfovibrio vulgaris cells with methyl viologen as an electron carrier. Anal. Chem. 1999, 71, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Lojou, E.; Durand, M.C.; Dolla, A.; Bianco, P. Hydrogenase activity control at Desulfovibrio vulgaris cell-coated carbon electrodes: Biochemical and chemical factors influencing the mediated bioelectrocatalysis. Electroanalysis 2002, 14, 913–922. [Google Scholar] [CrossRef]
- Geelhoed, J.S.; Stams, A.J.M. Electricity-assisted biological hydrogen production from acetate by Geobacter sulfurreducens. Environ. Sci. Technol. 2011, 45, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.D.; Siegert, M.; Logan, B.E. Hydrogen evolution catalyzed by viable and non-viable cells on biocathodes. Int. J. Hydrog. Energy 2014, 39, 16841–16851. [Google Scholar] [CrossRef]
- Aulenta, F.; Catapano, L.; Snip, L.; Villano, M.; Majone, M. Linking bacterial metabolism to graphite cathodes: Electrochemical insights into the H2-producing capability of Desulfovibrio sp. Chem. Sus. Chem. 2012, 5, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Jourdin, L.; Freguia, S.; Donose, B.C.; Keller, J. Autotrophic hydrogen-producing biofilm growth sustained by a cathode as the sole electron and energy source. Bioelectrochemistry 2015, 102, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, J.M.; Zaybak, Z.; Call, D.F.; Nam, J.Y.; Logan, B.E. Enrichment of microbial electrolysis cell biocathodes from sediment microbial fuel cell bioanodes. Appl. Environ. Microbiol. 2012, 78, 5212–5219. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Woodard, T.L.; Franks, A.E.; Summers, Z.M.; Lovley, D.R. Microbial electrosynthesis: Feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. MBio 2010, 1, e00103-10. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Hensley, S.A.; Franks, A.E.; Summers, Z.M.; Ou, J.; Woodard, T.L.; Snoeyenbos-West, O.L.; Lovley, D.R. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl. Environ. Microbiol. 2011, 77, 2882–2886. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Yumoto, I.; Kamagata, Y. Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl. Environ. Microbiol. 2015, 81, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Spirito, C.M.; Richter, H.; Rabaey, K.; Stams, A.J.M.; Angenent, L.T. Chain elongation in anaerobic reactor microbiomes to recover resources from waste. Curr. Opin. Biotechnol. 2014, 27, 115–122. [Google Scholar] [CrossRef] [PubMed]
- LaBelle, E.V.; Marshall, C.W.; Gilbert, J.A.; May, H.D. Influence of acidic pH on hydrogen and acetate production by an electrosynthetic microbiome. PLoS ONE 2014, 9, e109935. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Arends, J.B.A.; Vanwonterghem, I.; van Meerbergen, J.; Guo, K.; Tyson, G.W.; Rabaey, K. Selective enrichment establishes a stable performing community for microbial electrosynthesis of acetate from CO2. Environ. Sci. Technol. 2015, 49, 8833–8843. [Google Scholar] [CrossRef] [PubMed]
- Rago, L.; Ruiz, Y.; Baeza, J.A.; Guisasola, A.; Cortés, P. Microbial community analysis in a long-term membrane-less microbial electrolysis cell with hydrogen and methane production. Bioelectrochemistry 2015, 106, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, Y.; Lim, M.; Lee, H.; Lee, J.I.; Shin, W. Microbes as electrochemical CO2 conversion catalysts. ChemSusChem 2011, 4, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Park, J.-G.; Shin, W.-B.; Tian, D.-J.; Jun, H.-B. Microbial communities change in an anaerobic digestion after application of microbial electrolysis cells. Bioresour. Technol. 2017, 234, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Siegert, M.; Li, X.F.; Yates, M.D.; Logan, B.E. The presence of hydrogenotrophic methanogens in the inoculum improves methane gas production in microbial electrolysis cells. Front. Microbiol. 2015, 5, 778. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Sasaki, D.; Morita, M.; Hirano, S.; Matsumoto, N.; Ohmura, N.; Igarashi, Y. Bioelectrochemical system stabilizes methane fermentation from garbage slurry. Bioresour. Technol. 2010, 101, 3415–3422. [Google Scholar] [CrossRef] [PubMed]
- Babanova, S.; Carpenter, K.; Phadke, S.; Suzuki, S.; Ishii, S.; Phan, T.; Grossi-Soyster, E.; Flynn, M.; Hogan, J.; Bretschger, O. The effect of membrane type on the performance of microbial electrosynthesis cells for methane production. J. Electrochem. Soc. 2017, 164, H3015–H3023. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Quan, X.; Zhao, H. Evaluation on direct interspecies electron transfer in anaerobic sludge digestion of microbial electrolysis cell. Bioresour. Technol. 2016, 200, 235–244. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schuchmann, K.; Muller, V. Direct and reversible hydrogenation of CO2 to formate by a bacterial carbon dioxide reductase. Science 2013, 342, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.C.; Yoon, S.H.; Pan, M.; Burn, J.A.; Baliga, N.S.; Leigh, J.A. Effects of H2 and formate on growth yield and regulation of methanogenesis in Methanococcus maripaludis. J. Bacteriol. 2013, 195, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Ma, W.; Sun, J.; Sun, S.; Quan, X. Enriching functional microbes with electrode to accelerate the decomposition of complex substrates during anaerobic digestion of municipal sludge. Biochem. Eng. J. 2016, 111, 1–9. [Google Scholar] [CrossRef]
- McInerney, M.J.; Bryant, M.P. Anaerobic degradation of lactate by syntrophic associations of Methanosarcina barkeri and Desulfovibrio species and effect of H2 on acetate degradation. Appl. Environ. Microbiol. 1981, 41, 346–354. [Google Scholar] [PubMed]
- Holmes, D.E.; Chaudhuri, S.K.; Nevin, K.P.; Mehta, T.; Methé, B.A.; Liu, A.; Ward, J.E.; Woodard, T.L.; Webster, J.; Lovley, D.R. Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfurreducens. Environ. Microbiol. 2006, 8, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Lies, D.P.; Mielke, R.E.; Gralnick, J.A.; Newman, D.K. Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilm. Society 2005, 71, 4414–4426. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Sang, B.I. Extracellular electron transfer from cathode to microbes: Application for biofuel production. Biotechnol. Biofuels 2016, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Coursolle, D.; Baron, D.B.; Bond, D.R.; Gralnick, J.A. The MTR respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis. J. Bacteriol. 2010, 192, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G.; Nevin, K.P.; Nicoll, J.S.; Covalla, S.F.; Woodard, T.L.; Lovley, D.R. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 2006, 72, 7345–7348. [Google Scholar] [CrossRef] [PubMed]
- Freguia, S.; Masuda, M.; Tsujimura, S.; Kano, K. Lactococcus lactis catalyses electricity generation at microbial fuel cell anodes via excretion of a soluble quinone. Bioelectrochemistry 2009, 76, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Boon, N.; Aelterman, P.; Clauwaert, P.; de Schamphelaire, L.; Vanhaecke, L.; De Maeyer, K.; Höfte, M.; Verstraete, W.; Rabaey, K. Metabolites produced by Pseudomonas sp. enable a Gram-positive bacterium to achieve extracellular electron transfer. Appl. Microbiol. Biotechnol. 2008, 77, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Stams, A.J.M.; de Bok, F.A.M.; Plugge, C.M.; van Eekert, M.H.A.; Dolfing, J.; Schraa, G. Exocellular electron transfer in anaerobic microbial communities. Environ. Microbiol. 2006, 8, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Gorby, Y.A.; Yanina, S.; McLean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Kim, K.S.; et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lovley, D.R. Electricity production by geobacter sulfurreducens attached to electrodes electricity production by geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Bretschger, O.; Obraztsova, A.; Sturm, C.A.; In, S.C.; Gorby, Y.A.; Reed, S.B.; Culley, D.E.; Reardon, C.L.; Barua, S.; Romine, M.F.; et al. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl. Environ. Microbiol. 2007, 73, 7003–7012. [Google Scholar] [CrossRef] [PubMed]
- Meitl, L.A.; Eggleston, C.M.; Colberg, P.J.S.; Khare, N.; Reardon, C.L.; Shi, L. Electrochemical interaction of Shewanella oneidensis MR-1 and its outer membrane cytochromes OMCA and MTRC with hematite electrodes. Geochim. Cosmochim. Acta 2009, 73, 5292–5307. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Liu, Y.; Zhao, Z.; Zhao, Z.; Liu, S.; Zhao, H.; Quan, X. Enhancement of anaerobic methanogenesis at a short hydraulic retention time via bioelectrochemical enrichment of hydrogenotrophic methanogens. Bioresour. Technol. 2016, 218, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ. Microbiol. 2012, 14, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. R. Soc. Chem. 2012, 5, 8982–8989. [Google Scholar] [CrossRef]
- Philips, J.; Verbeeck, K.; Rabaey, K.; Arends, J.B.A. Electron Transfer Mechanisms in Biofilms. In Microbial Electrochemical and Fuel Cells; Woodhead Publishing: Cambridge, UK, 2016; Volume 3, pp. 67–113. [Google Scholar]
- Kouzuma, A.; Kato, S.; Watanabe, K. Microbial interspecies interactions: Recent findings in syntrophic consortia. Front. Microbiol. 2015, 6, 477. [Google Scholar] [CrossRef] [PubMed]
- Kato, S. Biotechnological aspects of microbial extracellular electron transfer. Microbes Environ. 2015, 30, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Call, D.F. Hardwiring microbes via direct interspecies electron transfer: Mechanisms and applications. Environ. Sci. Process. Impacts 2016, 18, 4158–4163. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.; Fan, W.; Nevin, K.P.; Lovley, D.R. Promoting interspecies electron transfer with biochar. Sci. Rep. 2014, 4, 5019. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hashimoto, K.; Watanabe, K. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc. Natl. Acad. Sci. USA 2012, 109, 10042–10046. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environ. Microbiol. 2015, 17, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Cruz Viggi, C.; Rossetti, S.; Fazi, S.; Paiano, P.; Majone, M.; Aulenta, F. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ. Sci. Technol. 2014, 48, 7536–7543. [Google Scholar] [CrossRef] [PubMed]
- Yamada, C.; Kato, S.; Ueno, Y.; Ishii, M.; Igarashi, Y. Conductive iron oxides accelerate thermophilic methanogenesis from acetate and propionate. J. Biosci. Bioeng. 2015, 119, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rotaru, A.-E.; Liu, F.; Philips, J.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresour. Technol. 2014, 173, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Wang, L.; Quan, X. Potential for direct interspecies electron transfer in an electric-anaerobic system to increase methane production from sludge digestion. Sci. Rep. 2015, 5, 11094. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Shrestha, P.M.; Walker, D.J.F.; Dang, Y.; Nevin, K.P.; Woodard, T.L.; Lovley, D.R. Metatranscriptomic evidence for direct interspecies electron transfer between geobacter and methanothrix species in methanogenic rice paddy soils. Appl. Environ. Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Nevin, K.P.; Woodard, T.L.; Mu, B.Z.; Lovley, D.R. Expanding the diet for DIET: Electron donors supporting direct interspecies electron transfer (DIET) in defined co-cultures. Front. Microbiol. 2016, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Happy together: Microbial communities that hook up to swap electrons. ISME J. 2016, 11, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Sydow, A.; Krieg, T.; Mayer, F.; Schrader, J.; Holtmann, D. Electroactive bacteria—Molecular mechanisms and genetic tools. Appl. Microbiol. Biotechnol. 2014, 98, 8481–8495. [Google Scholar] [CrossRef] [PubMed]
- Semenec, L.; Franks, A.E. Delving through electrogenic biofilms: From anodes to cathodes to microbes. AIMS Bioeng. 2015, 2, 222–248. [Google Scholar] [CrossRef]
- Shrestha, P.M.; Rotaru, A.E. Plugging in or going wireless: Strategies for interspecies electron transfer. Front. Microbiol. 2014, 5, 237. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Reach out and touch someone: Potential impact of DIET (direct interspecies energy transfer) on anaerobic biogeochemistry, bioremediation, and bioenergy. Rev. Environ. Sci. Biotechnol. 2011, 10, 101–105. [Google Scholar] [CrossRef]
- Malvankar, N.S.; Lovley, D.R. Microbial nanowires for bioenergy applications. Curr. Opin. Biotechnol. 2014, 27, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, P.C.; Grogger, M.; Veverka, D. Recent advances in microbial electrocatalysis. Electrocatalysis 2014, 5, 319–329. [Google Scholar] [CrossRef]
- Kracke, F.; Vassilev, I.; Krömer, J.O. Microbial electron transport and energy conservation—The foundation for optimizing bioelectrochemical systems. Front. Microbiol. 2015, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Schievano, A.; Pant, D. Electro-stimulated microbial factory for value added product synthesis. Bioresour. Technol. 2016, 213, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Harnisch, F. Is there a specific ecological niche for electroactive microorganisms? Chemelectrochem 2016, 3, 1–15. [Google Scholar] [CrossRef]
- Tan, Y.; Adhikari, R.Y.; Malvankar, N.S.; Pi, S.; Ward, J.E.; Woodard, T.L.; Nevin, K.P.; Xia, Q.; Tuominen, M.T.; Lovley, D.R. Synthetic biological protein nanowires with high conductivity. Small 2016, 12, 4481–4485. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Nevin, K.P. Electrobiocommodities: Powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr. Opin. Biotechnol. 2013, 24, 385–390. [Google Scholar] [CrossRef] [PubMed]
- ElMekawy, A.; Hegab, H.M.; Mohanakrishna, G.; Elbaz, A.F.; Bulut, M.; Pant, D. Technological advances in CO2 conversion electro-biorefinery: A step toward commercialization. Bioresour. Technol. 2016, 215, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.W.; LaBelle, E.V.; May, H.D. Production of fuels and chemicals from waste by microbiomes. Curr. Opin. Biotechnol. 2013, 24, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, J.; Lloyd, J.R.; Scott, K.; Premier, G.C.; Yu, E.H.; Curtis, T.; Head, I.M. A critical review of integration analysis of microbial electrosynthesis (MES) systems with waste biorefineries for the production of biofuel and chemical from reuse of CO2. Renew. Sustain. Energy Rev. 2016, 56, 116–132. [Google Scholar] [CrossRef]
- Persson, M.; Jonsson, O.; Wellinger, A. Biogas Upgrading To Vehicle Fuel Standards and Grid Injection. In Brochure of IEA Task 37. Energy from Biogas and Landfill Gas; International Energy Agency: Paris, France, 2006. [Google Scholar]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Dickinson, R.R.; Battye, D.L.; Linton, V.M.; Ashman, P.J.; Nathan, G.J. Alternative carriers for remote renewable energy sources using existing CNG infrastructure. Int. J. Hydrog. Energy 2010, 35, 1321–1329. [Google Scholar] [CrossRef]
- O’Shea, R.; Wall, D.; Kilgallon, I.; Murphy, J.D. Assessment of the impact of incentives and of scale on the build order and location of biomethane facilities and the feedstock they utilise. Appl. Energy 2016, 182, 394–408. [Google Scholar] [CrossRef]
- Budzianowski, W.M.; Brodacka, M. Biomethane storage: Evaluation of technologies, end uses, business models, and sustainability. Energy Convers. Manag. 2016. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, B.; Wang, G.; Guo, H. Methane formation route in the conversion of methanol to hydrocarbons. J. Energy Chem. 2014, 23, 201–206. [Google Scholar] [CrossRef]
- Jiang, Y.; Su, M.; Zhang, Y.; Zhan, G.; Tao, Y.; Li, D. Bioelectrochemical systems for simultaneously production of methane and acetate from carbon dioxide at relatively high rate. Int. J. Hydrog. Energy 2013, 38, 3497–3502. [Google Scholar] [CrossRef]
- Villano, M.; Scardala, S.; Aulenta, F.; Majone, M. Carbon and nitrogen removal and enhanced methane production in a microbial electrolysis cell. Bioresour. Technol. 2013, 130, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; San-Martín, M.I.; Escapa, A.; Morán, A. Domestic wastewater treatment in parallel with methane production in a microbial electrolysis cell. Renew. Energy 2016, 93, 442–448. [Google Scholar] [CrossRef]
- Villano, M.; Monaco, G.; Aulenta, F.; Majone, M. Electrochemically assisted methane production in a biofilm reactor. J. Power Sources 2011, 196, 9467–9472. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Fu, Q.; Kobayashi, H.; Ikarashi, M.; Wakayama, T.; Kawaguchi, H.; Vilcaez, J.; Maeda, H.; Sato, K. Electromethanogenic CO2 conversion by subsurface-reservoir microorganisms. Energy Procedia 2013, 37, 7014–7020. [Google Scholar] [CrossRef]
- Lu, L.; Ren, Z.J. Microbial electrolysis cells for waste biorefinery: A state of the art review. Bioresour. Technol. 2016, 215, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Zhu, X.; Zhang, L.; Tao, Y.; He, X.; Li, D.; Yan, Z. A new upgraded biogas production process: Coupling microbial electrolysis cell and anaerobic digestion in single-chamber, barrel-shape stainless steel reactor. Electrochem. Commun. 2014, 45, 67–70. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Chen, S.; Quan, X.; Yu, Q. Bioelectrochemical enhancement of anaerobic methanogenesis for high organic load rate wastewater treatment in a up-flow anaerobic sludge blanket (UASB) reactor. Sci. Rep. 2014, 4, 6658. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, L.; Chen, S.; Buisman, C.; ter Heijne, A. Bioelectrochemical enhancement of methane production in low temperature anaerobic digestion at 10 °C. Water Res. 2016, 99, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cai, W.; Guo, Z.; Wang, L.; Yang, C.; Varrone, C.; Wang, A. Microbial electrolysis contribution to anaerobic digestion of waste activated sludge, leading to accelerated methane production. Renew. Energy 2016, 91, 334–339. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, S.-H.; Park, H.-D. Enrichment of specific electro-active microorganisms and enhancement of methane production by adding granular activated carbon in anaerobic reactors. Bioresour. Technol. 2016, 205, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Han, T.; Guo, Z.; Varrone, C.; Wang, A.; Liu, W. Methane production enhancement by an independent cathode in integrated anaerobic reactor with microbial electrolysis. Bioresour. Technol. 2016, 208, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Zhu, X.; Zhan, G.; Bo, T.; Yang, Y.; Tao, Y.; He, X.; Li, D.; Yan, Z. Enhanced methane production in an anaerobic digestion and microbial electrolysis cell coupled system with co-cultivation of Geobacter and Methanosarcina. J. Environ. Sci. 2016, 42, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, J.; Xiao, B. Bioelectrochemical enhancement of hydrogen and methane production from the anaerobic digestion of sewage sludge in single-chamber membrane-free microbial electrolysis cells. Int. J. Hydrog. Energy 2013, 38, 1342–1347. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, D.; Dang, Y.; Chen, H.; Zhao, Z.; Zhang, Y.; Holmes, D.E. Stimulation of methanogenesis in anaerobic digesters treating leachate from a municipal solid waste incineration plant with carbon cloth. Bioresour. Technol. 2016, 222, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.C.; Feng, Q.; Ahn, Y. Performance of the bio-electrochemical anaerobic digestion of sewage sludge at different hydraulic retention times. Energy Fuels 2016, 30, 352–359. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.-C.; Bae, B.-U. Influence of applied voltage on the performance of bioelectrochemical anaerobic digestion of sewage sludge and planktonic microbial communities at ambient temperature. Bioresour. Technol. 2016, 220, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Gajaraj, S.; Huang, Y.; Zheng, P.; Hu, Z. Methane production improvement and associated methanogenic assemblages in bioelectrochemically assisted anaerobic digestion. Biochem. Eng. J. 2017, 117, 105–112. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Quan, X.; Zhang, J.; Zhao, H.; Chen, S. Effects of an electric field and zero valent iron on anaerobic treatment of azo dye wastewater and microbial community structures. Bioresour. Technol. 2011, 102, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Kobayashi, T.; Lu, X.; Kumar, G.; Xu, K. Biomethane recovery from Egeria densa in a microbial electrolysis cell-assisted anaerobic system: Performance and stability assessment. Chemosphere 2016, 149, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Zamalloa, C.; Arends, J.B.A.; Boon, N.; Verstraete, W. Performance of a lab-scale bio-electrochemical assisted septic tank for the anaerobic treatment of black water. New Biotechnol. 2013, 30, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, B.; Mehta, P.; Santoyo, G.; Roy, C.; Frigon, J.C.; Guiot, S.R. Electrolysis-enhanced co-digestion of switchgrass and cow manure. J. Chem. Technol. Biotechnol. 2014, 89, 1501–1506. [Google Scholar] [CrossRef]
- De Vrieze, J.; Gildemyn, S.; Arends, J.B.A.; Vanwonterghem, I.; Verbeken, K.; Boon, N.; Verstraete, W.; Tyson, G.W.; Hennebel, T.; Rabaey, K. Biomass retention on electrodes rather than electrical current enhances stability in anaerobic digestion. Water Res. 2014, 54, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, W.; Yang, C.; Wang, L.; Liang, B.; Thangavel, S.; Guo, Z.; Wang, A. Biocathodic methanogenic community in an integrated anaerobic digestion and microbial electrolysis system for enhancement of methane production from waste sludge. ACS Sustain. Chem. Eng. 2016, 4, 4913–4921. [Google Scholar] [CrossRef]
- Fu, Q.; Kobayashi, H.; Kawaguchi, H.; Vilcaez, J.; Sato, K. Identification of new microbial mediators for electromethanogenic reduction of geologically-stored carbon dioxide. Energy Procedia 2013, 37, 7006–7013. [Google Scholar] [CrossRef]
- Dang, Y.; Holmes, D.E.; Zhao, Z.; Woodard, T.L.; Zhang, Y.; Sun, D.; Wang, L.-Y.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, S.; Welte, C.; Li, X.; Oo, Y.M.; Kroeninger, L.; Heo, Y.; Zhang, M.; Ribeiro, D.; Lee, M.; Bhadbhade, M.; et al. Novel phenazine crystals enable direct electron transfer to methanogens in anaerobic digestion by redox potential modulation. Energy Environ. Sci. 2015, 9, 644–655. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Chen, S.; Quan, X. Enhanced production of methane from waste activated sludge by the combination of high-solid anaerobic digestion and microbial electrolysis cell with iron–graphite electrode. Chem. Eng. J. 2015, 259, 787–794. [Google Scholar] [CrossRef]
- Jenicek, P.; Keclik, F.; Maca, J.; Bindzar, J. Use of microaerobic conditions for the improvement of anaerobic digestion of solid wastes. Water Sci. Technol. 2008, 58, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Jenicek, P.; Koubova, J.; Bindzar, J.; Zabranska, J. Advantages of anaerobic digestion of sludge in microaerobic conditions. Water Sci. Technol. 2010, 62, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Ran, Z.; Gefu, Z.; Kumar, J.A.; Chaoxiang, L.; Xu, H.; Lin, L. Hydrogen and methane production in a bio-electrochemical system assisted anaerobic baffled reactor. Int. J. Hydrog. Energy 2014, 39, 13498–13504. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.A.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Call, D.; Logan, B.E. Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.M.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Rozendal, R.A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, A.W.; Hamelers, H.V.M.; Buisman, C.J. Microbial electrolysis cell with a microbial biocathode. Bioelectrochemistry 2010, 78, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Naraghi, Z.; Yaghmaei, S.; Mardanpour, M.M.; Hasany, M. Produced water treatment with simultaneous bioenergy production using novel bioelectrochemical systems. Electrochim. Acta 2015, 180, 535–544. [Google Scholar] [CrossRef]
- Marone, A.; Carmona-Martínez, A.A.; Sire, Y.; Meudec, E.; Steyer, J.P.; Bernet, N.; Trably, E. Bioelectrochemical treatment of table olive brine processing wastewater for biogas production and phenolic compounds removal. Water Res. 2016, 100, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Khalfbadam, H.M.; Ginige, M.P.; Sarukkalige, R.; Kayaalp, A.S.; Cheng, K.Y. Bioelectrochemical system as an oxidising filter for soluble and particulate organic matter removal from municipal wastewater. Chem. Eng. J. 2016, 296, 225–233. [Google Scholar] [CrossRef]
- Gil-Carrera, L.; Escapada, A.; Carracedo, B.; Morán, A.; Gómez, X. Performance of a semi-pilot tubular microbial electrolysis cell (MEC) under several hydraulic retention times and applied voltages. Bioresour. Technol. 2013, 146, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Yu, Q.; Ma, W.; Sun, J.; Quan, X. Enhanced decomposition of waste activated sludge via anodic oxidation for methane production and bioenergy recovery. Int. Biodeterior. Biodegrad. 2016, 106, 161–169. [Google Scholar] [CrossRef]
- Cerrillo, M.; Oliveras, J.; Viñas, M.; Bonmatí, A. Comparative assessment of raw and digested pig slurry treatment in bioelectrochemical systems. Bioelectrochemistry 2016, 110, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.Y.; Ho, G.; Cord-Ruwisch, R. Novel methanogenic rotatable bioelectrochemical system operated with polarity inversion. Environ. Sci. Technol. 2011, 45, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Katuri, K.P.; Werner, C.M.; Jimenez-Sandoval, R.J.; Chen, W.; Jeon, S.; Logan, B.E.; Lai, Z.; Amy, G.L.; Saikaly, P.E. A novel anaerobic electrochemical membrane bioreactor (AnEMBR) with conductive hollow-fiber membrane for treatment of low-organic strength solutions. Environ. Sci. Technol. 2014, 48, 12833–12841. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Song, Y.-C. Surface Modification of a Graphite fiber fabric anode for enhanced bioelectrochemical methane production. Energy Fuels 2016, 30, 6467–6474. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, L.; Wang, Q.; Quan, X.; Yang, J.; Chen, L. Cobalt recovery with simultaneous methane and acetate production in biocathode microbial electrolysis cells. Chem. Eng. J. 2014, 253, 281–290. [Google Scholar] [CrossRef]
- ElMekawy, A.; Srikanth, S.; Bajracharya, S.; Hegab, H.M.; Nigam, P.S.; Singh, A.; Mohan, S.V.; Pant, D. Food and agricultural wastes as substrates for bioelectrochemical system (BES): The synchronized recovery of sustainable energy and waste treatment. Food Res. Int. 2015, 73, 213–225. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Zeppilli, M.; Lai, A.; Villano, M.; Majone, M. Anion vs cation exchange membrane strongly affect mechanisms and yield of CO2 fixation in a microbial electrolysis cell. Chem. Eng. J. 2016, 304, 10–19. [Google Scholar] [CrossRef]
- Strong, P.J.; Xie, S.; Clarke, W.P. Methane as a resource: Can the methanotrophs add value? Environ. Sci. Technol. 2015, 49, 4001–4018. [Google Scholar] [CrossRef] [PubMed]
- Strong, P.J.; Kalyuzhnaya, M.; Silverman, J.; Clarke, W.P. A methanotroph-based biorefinery: Potential scenarios for generating multiple products from a single fermentation. Bioresour. Technol. 2016, 215, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Rostkowski, K.H.; Criddle, C.S.; Lepech, M.D. Cradle-to-gate life cycle assessment for a cradle-to-cradle cycle: Biogas-to-bioplastic (and back). Environ. Sci. Technol. 2012, 46, 9822–9829. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, J.; Fan, X.; Fu, S.; Sun, M.; Guo, R. Elimination of methane in exhaust gas from biogas upgrading process by immobilized methane-oxidizing bacteria. Bioresour. Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.C.; Torres, C.I. Critical transport rates that limit the performance of microbial electrochemistry technologies. Bioresour. Technol. 2016, 215, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, S.; Vanbroekhoven, K.; Buisman, C.J.; Pant, D.; Strik, D.P. Application of gas diffusion biocathode in microbial electrosynthesis from carbon dioxide. Environ. Sci. Pollut. Res. 2016, 23, 22292–22308. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, A.W.; Bergsma, J.; Kleijn, J.M.; Saakes, M.; Buisman, C.J.; Cohen Stuart, M.; Hamelers, H.V.M. Performance of metal alloys as hydrogen evolution reaction catalysts in a microbial electrolysis cell. Int. J. Hydrogen Energy 2011, 36, 10482–10489. [Google Scholar] [CrossRef]
- Fan, Y.; Han, S.-K.; Liu, H. Improved performance of CEA microbial fuel cells with increased reactor size. Energy Environ. Sci. 2012, 5, 8273–8280. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, H.; Zhou, S.; Shi, C.; Wang, C.; Ni, J. A novel UASB–MFC–BAF integrated system for high strength molasses wastewater treatment and bioelectricity generation. Bioresour. Technol. 2009, 100, 5687–5693. [Google Scholar] [CrossRef] [PubMed]
- Ballestra, P.; Cuq, J.-L. Influence of pressurized carbon dioxide on the thermal inactivation of bacterial and fungal spores. LWT Food Sci. Technol. 1998, 31, 84–88. [Google Scholar] [CrossRef]
- Strevett, K.A.; Vieth, R.F.; Grasso, D. Chemo-autotrophic biogas purification for methane enrichment: Mechanism and kinetics. Chem. Eng. J. Biochem. Eng. J. 1995, 58, 71–79. [Google Scholar] [CrossRef]
- Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A.; Heiszwolf, J.J. Multiphase monolith reactors: Chemical reaction engineering of segmented flow in microchannels. Chem. Eng. Sci. 2005, 60, 5895–5916. [Google Scholar] [CrossRef]
- Zhang, T.; Nie, H.; Bain, T.S.; Lu, H.; Cui, M.; Snoeyenbos-West, O.L.; Franks, A.E.; Nevin, K.; Russell, T.P.; Lovley, D. Improved cathode materials for microbial electrosynthesis. Energy Environ. Sci. 2013, 6, 217–224. [Google Scholar] [CrossRef]
- Aryal, N.; Halder, A.; Tremblay, P.-L.; Chi, Q.; Zhang, T. Enhanced microbial electrosynthesis with three-dimensional graphene functionalized cathodes fabricated via solvothermal synthesis. Electrochim. Acta 2016, 217, 117–122. [Google Scholar] [CrossRef]
- LaBarge, N.; Yilmazel, Y.D.; Hong, P.-Y.; Logan, B.E. Effect of pre-acclimation of granular activated carbon on microbial electrolysis cell startup and performance. Bioelectrochemistry 2017, 113, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Jourdin, L.; Freguia, S.; Flexer, V.; Keller, J. Bringing high-rate, CO2-based microbial electrosynthesis closer to practical implementation through improved electrode design and operating conditions. Environ. Sci Technol. 2016, 50, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Gildemyn, S.; Verbeeck, K.; Jansen, R.; Rabaey, K. The type of ion selective membrane determines stability and production levels of microbial electrosynthesis. Bioresour. Technol. 2016, 224, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Flores, G.; Poggi-Varaldo, H.M.; Solorza-Feria, O. Comparison of alternative membranes to replace high cost Nafion ones in microbial fuel cells. Int. J. Hydrog. Energy 2016, 21, 23354–23362. [Google Scholar] [CrossRef]
- Sleutels, T.H.J.A.; Hamelers, H.V.M.; Rozendal, R.A.; Buisman, C.J. Ion transport resistance in Microbial Electrolysis Cells with anion and cation exchange membranes. Int. J. Hydrog. Energy 2009, 34, 3612–3620. [Google Scholar] [CrossRef]
- Sleutels, T.H.J.A.; Lodder, R.; Hamelers, H.V.M.; Buisman, C.J. Improved performance of porous bio-anodes in microbial electrolysis cells by enhancing mass and charge transport. Int. J. Hydrog. Energy 2009, 34, 9655–9661. [Google Scholar] [CrossRef]
- Van Loosdrecht, M.C.M.; Lyklema, J.; Norde, W.; Zehnder, A.J.B. Bacterial adhesion: A physicochemical approach. Microb. Ecol. 1989, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rijnaarts, H.H.M.; Norde, W.; Bouwer, E.J.; Lyklema, J.; Zehnder, A.J.B. Reversibility and mechanism of bacterial adhesion. Colloids Surf. B Biointerfaces 1995, 4, 5–22. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, F.; Liu, J.; Zhang, X.; Huang, X.; Logan, B.E. Methane production in microbial reverse-electrodialysis methanogenesis cells (MRMCs) using thermolytic solutions. Environ. Sci. Technol. 2014, 48, 8911–8918. [Google Scholar] [CrossRef] [PubMed]
- Cusick, R.D.; Bryan, B.; Parker, D.S.; Merrill, M.D.; Mehanna, M.; Kiely, P.D.; Liu, G.; Logan, B.E. Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl. Microbiol. Biotechnol. 2011, 89, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, E.; Duquenne, F.; Rafrafi, Y.; Etcheverry, L.; Erable, B.; Bergel, A. Importance of the hydrogen route in up-scaling electrosynthesis for microbial CO2 reduction. Energy Environ. Sci. 2015, 8, 3731–3744. [Google Scholar] [CrossRef]
- May, H.D.; Marshall, C.W.; Labelle, E.V. Microbial Electrosynthetic Cells. U.S. Patent 20150259669 A1, 17 September 2015. [Google Scholar]
- Materi, W.P. Photoelectromethanogenic Microbial Fuel Cell for Co-Generation of Electricity and Methane from Carbon Dioxide. U.S. Patent 8,551,629 B2, 8 October 2013. [Google Scholar]
- Lovley, D.R.; Nevin, K. Microbial Production of Multi-Carbon Chemicals and Fuels from Water and Carbon Dioxide Using Electric Current. U.S. Patent 20,120,288,898 A1, 15 November 2012. [Google Scholar]
- Sefton, B. Chemoautotrophic Conversion of Carbon Oxides in Industrial Waste to Biomass and Chemical Products. U.S. Patent 9,206,451 B2, 8 December 2015. [Google Scholar]




| Reaction/Process | Type (Place) | References in Figure 3 | Microorganism [References] |
|---|---|---|---|
| BEC (C) | [1] | Methanobacterium palustre [8,19] | |
| Methanococcus maripaludis [48,49,50] | |||
| Methanobacterium-like (IM1) [51,52] | |||
| Methanosaeta spp. [34] | |||
| Methanosaeta concilii [33] | |||
| Methanosarcina barkeri [12,35] | |||
| Methanothermobacter thermautotrophicus [12,53] | |||
| Methanosaeta harundinacea [34] | |||
| Methanothermobacter sp. [54] | |||
| Methanoculleus sp. [54] | |||
| Methanobacterium sp. [14,54,55] | |||
| Methanosarcina mazei [56] | |||
| Methanothermobacter-like [15] | |||
| Methanobacteriaceae [20] | |||
| Methanobacterium petrolearium [33] | |||
| Methanobacterium subterraneum [33] | |||
| Methanothermobacter thermautotrophicus [9] | |||
| Methanosaeta concilii 2 [34] | |||
| BEC (C) | [2,3] | Desulfovibrio vulgaris [57,58] | |
| Geobacter sulfurreducens [12,59,60] | |||
| Pelobacter carbinolicus [35] | |||
| Hydrogenophaga caeni (EMB71) [19] | |||
| Desulfovibrio putealis (B7-43) [19] | |||
| Desulfovibrio paquesii [61] | |||
| Firmicutes [14,62] | |||
| Proteobacteria [62] | |||
| Bacteroidetes [62] | |||
| Actinobacteria [62] | |||
| Rhodococcus sp. [63] | |||
| Sphingobacteriales [55] | |||
| Desulfovibrio spp. [63] | |||
| BEC (C) | [4] | Sporomusa ovata [64] | |
| Sporomusa sphaeroides [65,66] | |||
| Sporomusa silvacetica [65] | |||
| Clostridium aceticum [65] | |||
| Clostridium ljungdahlii [65] | |||
| Moorella thermoacetica [65] | |||
| Clostridium thermoaceticum [67] | |||
| Acetobacterium spp. [42,55,68,69,70] | |||
| BEC (C) | [5] | Moorella thermoacetica [71] | |
| Clostridium formicoaceticum [71] | |||
| BC (C) | [6] | Methanobacterium sp. [14,43,55,72] | |
| Methanobacterium palustre [19] | |||
| Methanobacterium aarhusense [19] | |||
| Methanobacterium formicicum [72] | |||
| Methanobrevibacter arboriphilus [44,56] | |||
| Methanocorpusculum parvum [44] | |||
| Methanocorpusculum labreanum [63] | |||
| Methanobrevibacter [45,70,73] | |||
| Methanosarcina sp. [43] | |||
| Methanosarcina mazei [56] | |||
| Methanoculleus sp. [43,74] | |||
| Methanomicrobiales [20] | |||
| Methanobacterium petrolearium [33] | |||
| Methanobacterium subterraneum [33] | |||
| Methanothermobacter thermautotrophicus [9] | |||
| Methanothermobacter sp. [74] | |||
| Methanococcus maripaludis [47] | |||
| BC (C) | [7] | Acetobacterium woodii [65] | |
| Sporomusa silvacetica [65] | |||
| Clostridium aceticum [65] | |||
| Clostridium ljungdahlii [65] | |||
| Moorella thermoacetica [65] | |||
| Clostridium sp. [75] | |||
| BC (C) | [8] | Methanosaeta sp. [45,73,76] | |
| Methanosarcina sp. [7,74,77] | |||
| Methanosarcina thermophila [72] | |||
| Methanosaeta harundinacea [34] | |||
| Methanosarcina mazei [38,56] | |||
| BC (C) | [9] | Acetobacterium woodii [78] | |
| Candida boidinii [78] | |||
| BC (C) | [10] | Methanococcus maripaludis [79] | |
| Methanomicrobiales [20] | |||
| Methanobacterium formicicum [72] | |||
| BC (C) | n.s. | Acidovorax caeni (R-24608) [19] | |
| Hydrogenophaga caeni (EMB71) [19] | |||
| Methylocystis sp. (SC2) [19] | |||
| Unknown 1 | - | - | δ-Proteobacteria [14,45] |
| Geobacter sp. [54,76,80] | |||
| Pelobacter carbinolicus [35] | |||
| Desulfovibrio spp. [75,81] | |||
| Synergistetes-like [15,72] | |||
| Thermotogae-like [15] | |||
| Methylocystis sp. [14] | |||
| Unknown 2 | - | - | Methanospirillum hungatei [12] |
| Methanoregula boonei [12] | |||
| Methanocopusculum bavaricum [12] | |||
| Thermoplasma sp. [12] | |||
| Methanoculleus bourgensis [72] | |||
| Unknown 3 | - | - | Methanobacterium sp. (YCM1) [38] |
| Methanobacterium bryantii (RiH2) [38] | |||
| Methanosarcina mazei (Tuc01) [38] | |||
| Methanosarcina thermophila [72] | |||
| Methanobacterium arcticum (M2) [75] | |||
| Methanobacterium bryantii (MOH) [75] | |||
| Ch (A) | n.s. |
| Reactor Operation | Cathode Material | Cathode Potential (V vs. SHE) | Cathode Working Volume (mL) | Cathode Specific Surface (cm2) | Anode Reaction | Current Density (A·m−2) 5 | CH4 Yield (mmol·day−1·m−2) | CE (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| B | Carbon paper | −0.90 | 150 | 8 | WO | 0.69 (6) | 400 | 80 | [13] |
| B | Carbon black powder + Pt 1 | <−0.55 | 100 | 9.28 | n.r. | n.r. | 35.85 (2,3) | >100 | [11] |
| B | Graphite rod | <−0.4 | 350 | 13 | n.r. | 0.05 (8,9) | 3.5 | 80 | [52] |
| B | Graphite granules | −0.8 | 420 | 5700 | WO | 0.07 (10) | 5.1 | 75 | [14] |
| B | Carbon paper | −1.0 (4) | 10 | 3 | n.r. | 3.00 (8) | 87.9 | 19 | [53] |
| B | Carbon paper coated + carbon layer | −1.0 (4) | 10 | 3 | n.r. | n.r. | 95.5 | 96 | [9] |
| B | Graphite plate | −0.7 | 200 | 64.5 | BO | 1.00 (9) | 48.05 (2,3) | 83 | [33] |
| B | Carbon felt | −0.6/−0.7 | 240 | 98 | SO + BO | n.r. | 29.26 (2,3) | 51 | [44] |
| B | Granular graphite | −0.59 | 75 | n.r. | n.r. | n.r. | n.r. | 55 | [55] |
| B | Carbon felt | −0.95 | 240 | 49 | n.r. | n.r. | 1062 (2,3) | 56.7 | [137] |
| B | Carbon felt | −1.25 (4) | n.r. | 42 | BO | n.r. | >400 | >95 | [12] |
| B | Graphite felt | −1.5 (4) | 40 | 4700 | WO | n.r. | n.r. | n.r. | [27] |
| B | Graphite fiber brush | −0.439 | 120 | 13.8 | WO | 0.04 | 63.48 (3) | n.r. | [63] |
| B | Graphite bar | −0.5 | 100 | 8 | n.r. | n.r. | 0.22 | n.r. | [47] |
| C | Graphite felt | <−0.55 | n.r. | 250 | HO + WO | 0.21 (7,10) | 22.2 | 23 | [18] |
| C | Graphite granules | −0.93 | 860 | 11,094 | BO | 0.10 (8,10) | 8.84 (3) | 79 | [138,139] |
| C | Graphite granules | −0.8 | 420 | 5700 | WO | 0.20 (10) | 15.4 | 69 | [14] |
| C | Graphite plate | −0.7 | 800 | 64.5 | BO | >3 (9) | 155 (2,3) | >80 | [33] |
| C | Graphite felt | −0.7 | 240 | 250 | WO | 2.90 (7,10) | 477.7 (2,3) | <60 | [17] |
| C | Carbon cloth | −0.5 | 110 | 85.5 | n.r. | 0.04 (10) | 0.58 (2,3) | 63 | [38] |
| FB | Graphite plate | −0.6 | 250 | 4150 | WO | n.r. | 1901.19 (2,3) | n.r. | [74] |
| FB | Graphite granules | −0.85 | 860 | 11,094 | BO | 0.02 (9) | 1.58 (3) | 74 | [140] |
| FB | Graphite fiber brush | <−0.5 | 1750 | 947 | BO | 0.30 (8,10) | 200 | 96 | [8] |
| FB | Graphite felt | <−0.6 | 620 | 290 | HO + WO | 1.60 (7,10) | 205 (3) | 99 | [19] |
| FB | Carbon cloth | 0.8 (4) | 150 | 80 | BO | 0.17 (7,9) | 1103 | >90 | [15] |
| FB | Plain carbon felt | −0.75 (4) | 110 | 40 | BO | 2.60 (8,9) | 386 | 98 | [141] |
| FB | Carbon stick | −0.7 | 400 | 11 | WO | n.r. | 397 (3) | 24.2 | [10] |
| FB | Carbon stick + graphite felt 1 | −1.2 | 200 | 22 | WO | n.r. | 2911.99 (3) | 194.4 | [20] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blasco-Gómez, R.; Batlle-Vilanova, P.; Villano, M.; Balaguer, M.D.; Colprim, J.; Puig, S. On the Edge of Research and Technological Application: A Critical Review of Electromethanogenesis. Int. J. Mol. Sci. 2017, 18, 874. https://doi.org/10.3390/ijms18040874
Blasco-Gómez R, Batlle-Vilanova P, Villano M, Balaguer MD, Colprim J, Puig S. On the Edge of Research and Technological Application: A Critical Review of Electromethanogenesis. International Journal of Molecular Sciences. 2017; 18(4):874. https://doi.org/10.3390/ijms18040874
Chicago/Turabian StyleBlasco-Gómez, Ramiro, Pau Batlle-Vilanova, Marianna Villano, Maria Dolors Balaguer, Jesús Colprim, and Sebastià Puig. 2017. "On the Edge of Research and Technological Application: A Critical Review of Electromethanogenesis" International Journal of Molecular Sciences 18, no. 4: 874. https://doi.org/10.3390/ijms18040874
APA StyleBlasco-Gómez, R., Batlle-Vilanova, P., Villano, M., Balaguer, M. D., Colprim, J., & Puig, S. (2017). On the Edge of Research and Technological Application: A Critical Review of Electromethanogenesis. International Journal of Molecular Sciences, 18(4), 874. https://doi.org/10.3390/ijms18040874