Synthesis and Antiradical Activity of Isoquercitrin Esters with Aromatic Acids and Their Homologues
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Isoquercitrin Esters (2–5) by Enzymatic Approach
2.2. Preparation of Isoquercitrin Esters (6–11) by Chemical Approach
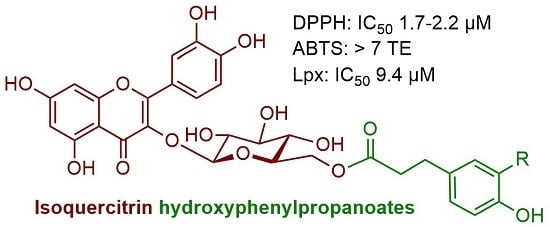
2.3. Antiradical Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. NMR and MS Methods
3.3. HPLC Analysis
3.4. Chemistry
3.4.1. Synthesis of Compounds 2–5
3.4.2. Synthesis of Compounds 32–34 (Structures in Figure S21 in the Supplementary Materials)
3.4.3. Synthesis of Compounds 35–40 (Structures in Figure S21 in the Supplementary Materials)
3.4.4. Synthesis of Compounds 6–11
3.5. Antioxidant Activity Measurement
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) |
| BnBr | Benzyl bromide |
| CAL-B | Lipase B from Candida antarctica |
| COSY | Correlation spectroscopy |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DCM | Dichloromethane |
| DMAP | 4-Dimethylaminopyridine |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| eq. | Equivalents |
| FCR | Folin-ciocalteau reduction |
| FDA | Food & drug administration |
| GAE | Gallic acid equivalents |
| GRAS | Generally recognized as safe |
| HMBC | Heteronuclear multiple-bond correlation spectroscopy |
| HSQC | Heteronuclear single-quantum correlation spectroscopy |
| IC50 | The concentration of the tested compound that inhibited the reaction by 50% |
| IQ | Isoquercitrin (quercetin-3-O-β-d-glucopyranoside) |
| Lpx | Lipid peroxidation |
| MS | Mass spectrometry |
| MS-ESI | Electron spray ionization mass spectrometry |
| NMR | Nuclear magnetic resonance |
| RT | Room temperature |
| TBARS | Thiobarbituric acid reactive substances |
| TBDMSCl | tert-Butyldimethylsilyl chloride |
| TE | Trolox-equivalents |
| THF | Tetrahydrofuran |
| TOCSY | Two-dimensional nuclear magnetic resonance spectroscopy |
| UV | Ultra violet |
References
- Wang, W.Y.; Sun, C.X.; Mao, L.K.; Ma, P.H.; Liu, F.G.; Yang, J.; Gao, Y.X. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- FDA. GRAS Notice for High Purity Quercetin. 2010. Available online: http://www.accessdata.fda.gov/scripts/fcn/gras_notices/grn341–1.pdf (accessed on 25 February 2014).
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Emura, K.; Yokomoto, A.; Toyoshi, T.; Moriwak, M. Effect of enzymatically modified isoquercitrin in spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2007, 53, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, A.P.; Kanashiro, A.; Fontanari, C.; da Silva, E.V.G.; Lucisano-Valim, Y.M.; Soares, E.G.; Faccioli, L.H. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Antidiabetic and antioxidative effects of Annona squamosa leaves are possibly mediated through quercetin-3-O-glucoside. Biofactors 2007, 31, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Gerstorferová, D.; Fliedrová, B.; Halada, P.; Marhol, P.; Křen, V.; Weignerová, L. Recombinant α-l-rhamnosidase from Aspergillus terreus in selective trimming of rutin. Process. Biochem. 2012, 47, 828–835. [Google Scholar] [CrossRef]
- Weignerová, L.; Marhol, P.; Gerstorferová, D.; Křen, V. Preparatory production of quercetin-3-β-d-glucopyranoside using alkali-tolerant thermostable α-l-rhamnosidase from Aspergillus terreus. Bioresour. Technol. 2012, 115, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Pišvejcová, A.; Křen, V.; Lama, M.; Riva, S. Generation of an α-l-rhamnosidase library and its application for the selective derhamnosylation of natural products. Biotechnol. Bioeng. 2004, 87, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Nakajima, N. Structural aspects of acylated plant pigments: Stabilization of flavonoid glucosides and interpretation of their functions. J. Mol. Catal. B Enzym. 2003, 23, 411–417. [Google Scholar] [CrossRef]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2011, 28, 1626–1695. [Google Scholar] [CrossRef] [PubMed]
- Bloor, S.J. Deep blue anthocyanins from blue Dianella berries. Phytochemistry 2001, 58, 923–927. [Google Scholar] [CrossRef]
- Alluis, B.; Dangles, O. Acylated flavone glucosides: Synthesis, conformational investigation, and complexation properties. Helv. Chim. Acta 1999, 82, 2201–2212. [Google Scholar] [CrossRef]
- Matsuda, H.; Ninomiya, K.; Shimoda, H.; Yoshikawa, M. Hepatoprotective principles from the flowers of Tilia argentea (Linden): Structure requirements of tiliroside and mechanisms of action. Bioorg. Med. Chem. 2002, 10, 707–712. [Google Scholar] [CrossRef]
- Vavříková, E.; Langschwager, F.; Ježová-Kalachová, L.; Křenková, A.; Mikulová, B.; Kuzma, M.; Křen, V.; Valentová, K. Isoquercitrin esters with mono- or dicarboxylic acids: Enzymatic preparation and properties. Int. J. Mol. Sci. 2016, 17, 899. [Google Scholar] [CrossRef] [PubMed]
- Danieli, B.; Bertario, A. Chemoenzymatic synthesis of 6″-O-(3-arylprop-2-enoyl) derivatives of the flavonol glucoside isoquercitrin. Helv. Chim. Acta 1993, 76, 2981–2991. [Google Scholar] [CrossRef]
- Nakajima, N.; Ishihara, K.; Hamada, H.; Kawabe, S.; Furuya, T. Regioselective acylation of flavonoid glucoside with aromatic acid by an enzymatic reaction system from cultured cells of Ipomoea batatas. J. Biosci. Bioeng. 2000, 90, 347–349. [Google Scholar] [CrossRef]
- Kajjout, M.; Rolando, C. Regiospecific synthesis of quercetin O-β-d-glucosylated and O-β-d-glucuronidated isomers. Tetrahedron 2011, 67, 4731–4741. [Google Scholar] [CrossRef]
- Ren, X.H.; Shen, L.L.; Muraoka, O.; Cheng, M.S. Synthesis of quercetin 3-O[6″-O-(trans-p-coumaroyl)]-β-d-glucopyranoside. J. Carbohydr. Chem. 2011, 30, 119–131. [Google Scholar] [CrossRef]
- Gažák, R.; Purchartová, K.; Marhol, P.; Živná, L.; Sedmera, P.; Valentová, K.; Kato, N.; Matsumura, H.; Kaihatsu, K.; Křen, V. Antioxidant and antiviral activities of silybin fatty acid conjugates. Eur. J. Med. Chem. 2010, 45, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Mastihubová, M.; Mastihuba, V. Donor specificity and regioselectivity in Lipolase mediated acylations of methyl α-d-glucopyranoside by vinyl esters of phenolic acids and their analogues. Bioorg. Med. Chem. Lett. 2013, 23, 5389–5392. [Google Scholar] [CrossRef] [PubMed]
- Krejzová, J.; Šimon, P.; Vavříková, E.; Slámová, K.; Pelantová, H.; Riva, S.; Spiwok, V.; Křen, V. Enzymatic synthesis of new C-6-acylated derivatives of NAG-thiazoline and evaluation of their inhibitor activities towards fungal β-N-acetylhexosaminidase. J. Mol. Catal. B Enzym. 2013, 87, 128–134. [Google Scholar] [CrossRef]
- Nakajima, N.; Ishihara, K.; Itoh, T.; Furuya, T.; Hamada, H. Lipase-catalyzed direct and regioselective acylation of flavonoid glucoside for mechanistic investigation of stable plant pigments. J. Biosci. Bioeng. 1999, 87, 105–107. [Google Scholar] [CrossRef]
- Wang, L.Y.; Tsai, H.Y.; Lin, H.C. Novel supramolecular side-chain banana-shaped liquid crystalline polymers containing covalent- and hydrogen-bonded bent bores. Macromolecules 2010, 43, 1277–1288. [Google Scholar] [CrossRef]
- Vitoriano, B.C.; Carvalho, L.C.R.; Estevao, M.S.; Sekera, M.H.; Marques, M.M.B. A simple route toward new clomiphene metabolites. Synlett 2010, 5, 753–756. [Google Scholar] [CrossRef]
- Catel, Y.; Aladedunye, F.; Przybylski, R. Synthesis, radical scavenging activity, protection during storage, and frying by novel antioxidants. J. Agric. Food Chem. 2010, 58, 11081–11089. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, W.Q.; Wang, L.Y.; Shan, L.; Li, H.L.; Huang, J.; Sun, Q.Y.; Zhang, W.D. Synthesis of derivatives of methyl rosmarinate and their inhibitory activities against matrix metalloproteinase-1 (MMP-1). Eur. J. Med. Chem. 2013, 62, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Zhao, P.; Hu, J.L.; Liu, J.H.; Liu, Y.; Wang, Z.Q.; Xia, Y.F.; Dai, Y.; Chen, L. Synthesis, in vitro and in vivo antitumor activity of scopoletin-cinnamic acid hybrids. Eur. J. Med. Chem. 2015, 93, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, Q.U.; Yang, M.T.; Memon, K.H.; Lateef, M.; Na, D.; Wan, S.B.; Eric, D.; Zhang, L.J.; Jiang, T. 1,2,3,4,6-Pentakis[-O-(3,4,5-trihydroxybenzoyl)]-α,β-d-glucopyranose (PGG) analogs: Design, synthesis, anti-tumor and anti-oxidant activities. Carbohydr. Res. 2016, 430, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, S.; Ramachandra, M.S.; Krishnaraju, A.V.; Trimurtulu, G.; Subbaraju, G.V. Antioxidant and antimicrobial activity evaluation of polyhydroxycinnamic acid ester derivatives. Indian J. Chem. B 2006, 45, 252–257. [Google Scholar] [CrossRef]
- Pyszková, M.; Biler, M.; Biedermann, D.; Valentová, K.; Kuzma, M.; Vrba, J.; Ulrichová, J.; Sokolová, R.; Mojovic, M.; Popovic-Bijelic, A.; et al. Flavonolignan 2,3-dehydroderivatives: Preparation, antiradical and cytoprotective activity. Free Radic. Biol. Med. 2016, 90, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, A.; Rhim, T.J.; Choi, M.Y.; Choi, J.S.; Kim, Y.C.; Kim, M.S.; Park, H.J. Simultaneous analysis and peroxynitrite-scavenging activity of galloylated flavonoid glycosides and ellagic acid in Euphorbia supina. Arch. Pharm. Res. 2014, 37, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Iritani, K.; Yonemori, S.; Oyama, Y.; Takeda, Y. Isolation and antioxidant activity of galloyl flavonol glycosides from the seashore plant, Pemphis acidula. Biosci. Biotechnol. Biochem. 2001, 65, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, I.J.; Lee, H.Y.; Hwang, B.Y.; Han, S.B.; Kim, Y. Distinct inhibitory mechanisms of isoquercitrin gallate and its aglycone on zymosan-induced peroxynitrite production in macrophages. Nitric Oxide 2007, 17, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Factors influencing the antioxidant activity determined by the ABTS+ radical cation assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Joyeux, M.; Lobstein, A.; Anton, R.; Mortier, F. Comparative antilipoperoxidant, antinecrotic and scavenging properties of terpenes and biflavones from ginkgo and some flavonoids. Planta Med. 1995, 61, 126–129. [Google Scholar] [CrossRef] [PubMed]







| Compounds | FCR (GAE) | DPPH (IC50, µM) | ABTS (TE) | Lpx (IC50, µM) |
|---|---|---|---|---|
| Isoquercitrin (1, IQ) | 1.11 ± 0.30 a | 1.40 ± 0.06 d | 1.98 ± 0.07 f | 972 ± 11 |
| IQ benzoate (2) | 1.24 ± 0.05 a | 2.85 ± 0.22 | 7.34 ± 0.01 g | 8.29 ± 0.39 h |
| IQ phenylacetate (3) | 1.45 ± 0.03 b | 1.89 ± 0.04 d | 1.10 ± 0.20 | 6.68 ± 0.39 i |
| IQ phenylpropanoate (4) | 1.47 ± 0.04 b | 3.31 ± 0.22 | 3.65 ± 0.18 | 10.5 ± 0.7 |
| IQ cinnamate (5) | 0.51 ± 0.13 c | 4.64 ± 0.16 | 0.64 ± 0.05 | 295 ± 14 |
| IQ 4-OH benzoate (6) | 0.90 ± 0.05 a | 11.5 ± 0.4 | 1.81 ± 0.25 f | 12.6 o ± 0.3 j |
| IQ vanillate (7) | 1.50 ± 0.04 b | 2.18 ± 0.04 e | 7.05 ± 0.07 g | 14.1 ± 0.6 j |
| IQ gallate (8) | 0.52 ± 0.15 c | 2.28 ± 0.18 e | 5.27 ± 0.20 | 6.64 ± 0.27 i |
| IQ 4-OHPh propanoate (9) | 0.59 ± 0.06 c | 1.77 ± 0.06 d | 7.18 ± 0.07 g | 9.39 ± 0.29 h |
| IQ 3,4-diOHPh propanoate (10) | 0.88 ± 0.04 a | 1.67 ± 0.09 d | 7.20 ± 0.15 g | 9.41 ± 0.28 h |
| IQ 4-OH-3-OMePh propanoate (11) | 1.48 ± 0.03 b | 2.22 ± 0.11 e | 7.33 ± 0.05 g | 9.36 ± 0.22 h |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heřmánková-Vavříková, E.; Křenková, A.; Petrásková, L.; Chambers, C.S.; Zápal, J.; Kuzma, M.; Valentová, K.; Křen, V. Synthesis and Antiradical Activity of Isoquercitrin Esters with Aromatic Acids and Their Homologues. Int. J. Mol. Sci. 2017, 18, 1074. https://doi.org/10.3390/ijms18051074
Heřmánková-Vavříková E, Křenková A, Petrásková L, Chambers CS, Zápal J, Kuzma M, Valentová K, Křen V. Synthesis and Antiradical Activity of Isoquercitrin Esters with Aromatic Acids and Their Homologues. International Journal of Molecular Sciences. 2017; 18(5):1074. https://doi.org/10.3390/ijms18051074
Chicago/Turabian StyleHeřmánková-Vavříková, Eva, Alena Křenková, Lucie Petrásková, Christopher Steven Chambers, Jakub Zápal, Marek Kuzma, Kateřina Valentová, and Vladimír Křen. 2017. "Synthesis and Antiradical Activity of Isoquercitrin Esters with Aromatic Acids and Their Homologues" International Journal of Molecular Sciences 18, no. 5: 1074. https://doi.org/10.3390/ijms18051074
APA StyleHeřmánková-Vavříková, E., Křenková, A., Petrásková, L., Chambers, C. S., Zápal, J., Kuzma, M., Valentová, K., & Křen, V. (2017). Synthesis and Antiradical Activity of Isoquercitrin Esters with Aromatic Acids and Their Homologues. International Journal of Molecular Sciences, 18(5), 1074. https://doi.org/10.3390/ijms18051074