Effects of Gelatin Hydrogel Containing Anti-Transforming Growth Factor-β Antibody in a Canine Filtration Surgery Model
Abstract
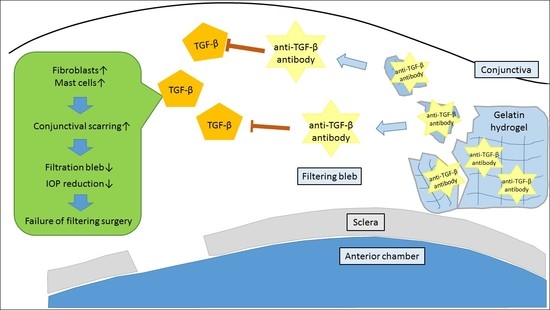
:1. Introduction
2. Results
2.1. Verification of Anti-TGF-β Antibody in GH
2.2. IOP Change
2.3. Bleb Score
2.4. Subconjunctival/Scleral Area Ratio
2.5. Vimentin-Positive Cells
2.6. TGF-β Antibody-Positive Cells, Proliferative Cell Nuclear Antigen (PCNA)-Positive Cells, and Mast Cells
3. Discussion
4. Materials and Methods
4.1. Verification of Anti-TGF-β Antibody in GH
4.2. Animals and IOP Measurement
4.3. Glaucoma Filtration Surgery Model
4.4. Experiment Protocol
4.5. Bleb Scores
4.6. Histological Examination
4.7. Masked Manner
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Skuta, G.L.; Parrish, R.K. Wound healing in glaucoma filtering surgery. Surv. Ophthalmol. 1987, 32, 149–170. [Google Scholar] [CrossRef]
- Tripathi, R.C.; Li, J.; Chalam, K.V.; Tripathi, B.J. Expression of growth factor mRNAs by human Tenon’s capsule fibroblasts. Exp. Eye Res. 1996, 63, 339–346. [Google Scholar] [PubMed]
- Lama, P.J.; Fechtner, R.D. Antifibrotics and wound healing in glaucoma surgery. Surv. Ophthalmol. 2003, 48, 314–346. [Google Scholar] [CrossRef]
- Mochizuki, K.; Jikihara, S.; Ando, Y.; Hori, N.; Yamamoto, T.; Kitazawa, Y. Incidence of delayed onset infection after trabeculectomy with adjunctive mitomycin C or 5-fluorouracil treatment. Br. J. Ophthalmol. 1997, 81, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, E.J.; Stevens, R.K.; Musch, D.C.; Karp, K.O.; Lichter, P.R.; Bergstrom, T.J.; Skuta, G.L. Bleb-related endophthalmitis after trabeculectomy with mitomycin C. Ophthalmology 1996, 103, 650–656. [Google Scholar] [PubMed]
- Greenfield, D.S.; Suner, I.J.; Miller, M.P.; Kangas, T.A.; Palmberg, P.F.; Flynn, H.W., Jr. Endophthalmitis after filtering surgery with mitomycin. Arch. Ophthalmol. 1996, 114, 943–949. [Google Scholar] [PubMed]
- Shah, M.; Foreman, D.M.; Ferguson, M.W. Neutralisation of TGF-β1 and TGF-β2 or exogenous addition of TGF-β3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 1995, 108, 985–1002. [Google Scholar] [PubMed]
- Levine, J.H.; Moses, H.L.; Gold, L.I.; Nanney, L.B. Spatial and temporal patterns of immunoreactive transforming growth factor-β1, -β2 and -β3 during excisional wound repair. Am. J. Pathol. 1993, 143, 368–380. [Google Scholar] [PubMed]
- Lutty, G.A.; Merfes, C.; Threlkeld, A.B.; Crone, S.; Mcleod, D.S. Heterogeneity in localization of isoforms of TGF-β in human retina, vitreous and choroid. Investig. Ophthalmol. Vis. Sci. 1993, 34, 477–487. [Google Scholar]
- Pasquale, L.R.; Dorman-Pease, M.E.; Lutty, G.A.; Quigley, H.A.; Jampel, H.D. Immunolocalisation of TGF-β1, TGF-β2 and TGF-β3 in the anterior segment of the human eye. Investig. Ophthalmol. Vis. Sci. 1993, 34, 23–30. [Google Scholar]
- Jampel, H.D.; Roche, N.; Stark, W.J.; Roberts, A.B. Transforming growth factor-β in human aqueous humor. Curr. Eye Res. 1990, 9, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.F.; Reichel, M.B.; Gay, J.A.; D’Esposita, F.; Alexander, R.A.; Khaw, P.T. Transforming growth factor-β1, -β2 and -β3 in vivo: Effects on normal and mitomycin-C modulated conjunctival scarring. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1975–1982. [Google Scholar]
- Tripathi, R.C.; Ki, J.; Chan, W.F. Aqueous humor in glaucomatous eyes contains an increased level of TGF2. Exp. Eye Res. 1994, 59, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Picht, G.; Welge-Luessen, U.; Grehn, F.; Lütjen-Drecoll, E. Transforming growth factor β2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefe’s Arch. Clin. Exp. Ophthalmol. 2001, 239, 199–207. [Google Scholar] [CrossRef]
- Cordeiro, M.F. Beyond mitomycin: TGF-β and wound healing. Prog. Retin. Eye Res. 2002, 21, 75–89. [Google Scholar] [CrossRef]
- Zhu, X.; Li, L.; Zou, L.; Zhu, X.; Xian, G.; Li, H.; Tan, Y.; Xie, L. A novel aptamer targeting TGF-β receptor II inhibits transdifferentiation of human tenon’s fibroblasts into myofibroblast. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6897–6903. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, D.; Zhu, X.; Li, L.; Li, H.; Gou, F.; Chen, X.; Tan, Y.; Xie, L. Evaluation of chitosan/aptamer targeting TGF-β receptor II thermo-sensitive gel for scarring in rat glaucoma filtration surgery. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5465–5476. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Ter-Vehn, T.; Sieprath, S.; Katzenberger, B.; Gebhardt, S.; Grehn, F.; Schlunck, G. Contractility as a prerequisite for TGF-β-induced myofibroblast transdifferentiation in human tenon fibroblasts. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4895–4904. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.F. Role of transforming growth factor β in conjunctival scarring. Clin. Sci. 2003, 104, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Branton, M.H.; Kopp, J.B. TGF-β and fibrosis. Microbes Infect. 1999, 1, 1349–1365. [Google Scholar] [CrossRef]
- Freedman, J. TGF-β2 antibody in trabeculectomy. Ophthalmology 2009, 116, 166. [Google Scholar] [CrossRef] [PubMed]
- Mead, A.L.; Wong, T.T.L.; Cordeiro, M.F.; Anderson, I.K.; Khaw, P.T. Evaluation of anti-TGF-β2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3394–3401. [Google Scholar] [CrossRef]
- CAT-152 0102 Trabeculectomy Study Group; Khaw, P.; Grehn, F.; Hollo, G.; Overton, B.; Wilson, R.; Vogel, R.; Smith, Z. A phase III study of subconjunctival human anti-transforming growth factor β2 monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology 2007, 114, 1822–1830. [Google Scholar] [PubMed]
- Blandford, D.L.; Smith, T.J.; Brown, J.D.; Pearson, P.A.; Ashton, P. Subconjunctival sustained release 5-fluorouracil. Investig. Ophthalmol. Vis. Sci. 1992, 33, 3430–3435. [Google Scholar]
- Min, J.K.; Kee, C.W.; Sohn, S.; Lee, H.J.; Woo, J.M.; Yim, J.H. Surgical outcome of mitomycin C-soaked collagen matrix implant in trabeculectomy. J. Glaucoma 2013, 22, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.C.; Bai, Y.J.; Tian, Z.; Hu, H.Y.; You, X.H.; Lin, J.X.; Liu, S.R.; Zhuo, Y.H.; Luo, R.J. Anti-proliferation effects of Sirolimus sustained delivery film in rabbit glaucoma filtration surgery. Mol. Vis. 2011, 17, 2495–2506. [Google Scholar] [PubMed]
- Tabata, Y. Biomaterial technology for tissue engineering applications. J. R. Soc. Interface 2009, 6, S311–S324. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Hijikata, S.; Ikada, Y. Enhanced vascularization and tissue granulation by basic fibroblast growth factor impregnated in gelatin hydrogels. J. Control. Release 1994, 31, 189–199. [Google Scholar] [CrossRef]
- Yamamoto, M.; Tabata, Y.; Hong, L.; Miyamoto, S.; Hashimoto, N.; Ikada, Y. Bone regeneration by transforming growth factor β1 released from a biodegradable hydrogel. J. Control. Release 2000, 64, 133–142. [Google Scholar] [CrossRef]
- Kawanaka, H.; Takagi, G.; Miyamoto, M.; Tara, S.; Takagi, I.; Takano, H.; Yasutake, M.; Tabata, Y.; Mizuno, K. Therapeutic angiogenesis by controlled-release fibroblast growth factor in a patient with Chung-Strauss syndrome complicated by an intractable ischemic leg ulcer. Am. J. Med. Sci. 2009, 338, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Koyama, H.; Miyata, T.; Hosaka, A.; Tabata, Y.; Takato, T.; Nagawa, H. Selective and sustained delivery of basic fibroblast growth factor (bFGF) for treatment of peripheral arterial disease: Results of a phase I trial. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Sakamoto, T.; Hinton, D.R.; Spee, C.; Ogura, Y.; Tabata, Y.; Ikada, Y.; Ryan, S.J. A new model of subretinal neovascularization in the rabbit. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2110–2119. [Google Scholar]
- Yang, C.F.; Yasukawa, T.; Kimura, H.; Miyamoto, H.; Honda, Y.; Tabata, Y.; Ikada, Y.; Ogura, Y. Experimental corneal neovascularization by basic fibroblast growth factor incorporated into gelatin hydrogel. Ophthalmic Res. 2000, 32, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Sugiyama, T.; Takai, S.; Jin, D.; Shibata, M.; Oku, H.; Tabata, Y.; Ikeda, T. Effects of gelatin hydrogel containing chymase inhibitor on scarring in a canine filtration surgery model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7672–7680. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Sugiyama, T.; Takai, S.; Jin, D.; Ueki, M.; Oku, H.; Tabata, Y.; Ikeda, T. Effects of gelatin hydrogel loading mitomycin C on conjunctival scarring in a canine filtration surgery model. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2601–2605. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y.; Tabata, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar] [PubMed]
- Perkins, T.W.; Faha, B.; Ni, M.; Kiland, J.A.; Poulsen, G.L.; Antelman, D.; Atencio, I.; Shinoda, J.; Sinha, D.; Brumback, L.; et al. Adenovirus-mediated gene therapy using human p21WAF-1/Cip-1 to prevent wound healing in a rabbit model of glaucoma filtration surgery. Arch. Ophthalmol. 2002, 120, 941–949. [Google Scholar] [CrossRef] [PubMed]








| Indexes | GH-TGF-β Group | GH Group | p-Value (Student’s t-Test) |
|---|---|---|---|
| Ratio of the conjunctival area to the scleral area | 1.0 ± 0.1 | 2.4 ± 0.1 | 0.001 |
| Density of fibroblasts, per mm2 | 27.8 ± 8.6 | 67.6 ± 18.7 | 0.01 |
| Density of TGF-β-positive cells, per mm2 | 9.8 ± 1.5 | 18.2 ± 3.3 | 0.04 |
| Density of PCNA-positive cells, per mm2 | 4.2 ± 3.2 | 14.4 ± 6.0 | 0.03 |
| Density of mast cells, per mm2 | 7.2 ± 1.6 | 13.8 ± 2.0 | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, M.; Kojima, S.; Sugiyama, T.; Jin, D.; Takai, S.; Oku, H.; Kohmoto, R.; Ueki, M.; Ikeda, T. Effects of Gelatin Hydrogel Containing Anti-Transforming Growth Factor-β Antibody in a Canine Filtration Surgery Model. Int. J. Mol. Sci. 2017, 18, 985. https://doi.org/10.3390/ijms18050985
Maeda M, Kojima S, Sugiyama T, Jin D, Takai S, Oku H, Kohmoto R, Ueki M, Ikeda T. Effects of Gelatin Hydrogel Containing Anti-Transforming Growth Factor-β Antibody in a Canine Filtration Surgery Model. International Journal of Molecular Sciences. 2017; 18(5):985. https://doi.org/10.3390/ijms18050985
Chicago/Turabian StyleMaeda, Michiko, Shota Kojima, Tetsuya Sugiyama, Denan Jin, Shinji Takai, Hidehiro Oku, Ryohsuke Kohmoto, Mari Ueki, and Tsunehiko Ikeda. 2017. "Effects of Gelatin Hydrogel Containing Anti-Transforming Growth Factor-β Antibody in a Canine Filtration Surgery Model" International Journal of Molecular Sciences 18, no. 5: 985. https://doi.org/10.3390/ijms18050985
APA StyleMaeda, M., Kojima, S., Sugiyama, T., Jin, D., Takai, S., Oku, H., Kohmoto, R., Ueki, M., & Ikeda, T. (2017). Effects of Gelatin Hydrogel Containing Anti-Transforming Growth Factor-β Antibody in a Canine Filtration Surgery Model. International Journal of Molecular Sciences, 18(5), 985. https://doi.org/10.3390/ijms18050985