Suppression of Osteoclastogenesis by Melatonin: A Melatonin Receptor-Independent Action
Abstract
:1. Introduction
2. Results
2.1. Melatonin Inhibited Receptor Activator of Nuclear Factor κB Ligand (RANKL)-Induced Osteoclastogenesis from Mouse Bone-Marrow Derived Macrophages (BMMs)
2.2. Type 1a (MT1) and Type 1b (MT2) Melatonin Receptors are Expressed in Osteoclast Precursor Cells
2.3. The Silencing of MT1/MT2 Receptors Failed to Reverse the Anti-Osteoclastogenic Effect of Melatonin
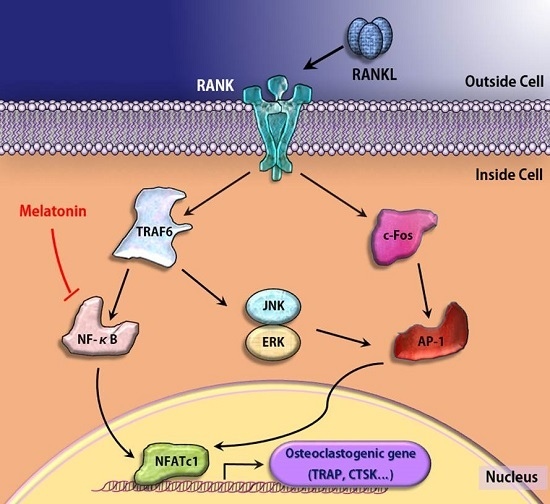
2.4. Melatonin Had Little Effect on the RANKL-Induced Activation of Three Major Mitogen-Activated Protein Kinases (MAPKs) but Markedly Blocked Nuclear Factor of Activated T Cell Cytoplasmic 1 (NFATc1) Induction
2.5. Melatonin Inhibits NF-κB Activity in RANKL-Stimulated Bone Marrow-Derived Macrophages (BMMs)
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Isolation of Macrophages from Mouse Bone Marrow and In Vitro Osteoclast Differentiation
4.3. Cytotoxicity Measurement and Cell Proliferation Assay
4.4. Gene Knockdown by siRNA Transfection
4.5. Reverse Transcriptase Polymerase-Chain Reaction (RT-PCR) and Real-Time PCR Analyses
4.6. Western Blotting
4.7. NF-κB Luciferase Reporter Assay
4.8. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RANKL | Receptor activator of nuclear factor κB ligand |
| M-CSF | Macrophage-colony stimulating factor |
| BMM | Bone marrow-derived macrophage |
| TRAP | Tartrate-resistant acid phosphatase |
References
- Opie, L.H.; Lecour, S. Melatonin has multiorgan effects. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Soru, C.; Pluchino, R.; Intra, C.; Iriti, M. The impact of melatonin in research. Molecules 2016, 21, 240. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumai, S.R.; Srinivasan, V.; Maestroni, G.J.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006, 273, 2813–2838. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Emet, M.; Ozcan, H.; Ozel, L.; Yayla, M.; Halici, Z.; Hacimuftuoglu, A. A review of melatonin, its receptors and drugs. Eurasian J. Med. 2016, 48, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.R.; Gonzalez-Yanes, C.; Maldonado, M.D. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, Y.; Fan, C.; Han, J.; Wang, D.; Di, S.; Hu, W.; Liu, D.; Li, X.; Reiter, R.J.; et al. Melatonin as a potential anticarcinogen for non-small-cell lung cancer. Oncotarget 2016, 7, 46768–46784. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Kashyap, D.; Sharma, A.K.; Sandhu, S.S. Molecular aspects of melatonin (MLT)-mediated therapeutic effects. Life Sci. 2015, 135, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Von Gall, C.; Stehle, J.H.; Weaver, D.R. Mammalian melatonin receptors: Molecular biology and signal transduction. Cell Tissue Res. 2002, 309, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C. Melatonin receptors in humans: Biological role and clinical relevance. Biomed. Pharmacother. 2006, 60, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Kular, J.; Tickner, J.; Chim, S.M.; Xu, J. An overview of the regulation of bone remodelling at the cellular level. Clin. Biochem. 2012, 45, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Kim, N.; Kadono, Y.; Rho, J.; Lee, S.Y.; Lorenzo, J.; Choi, Y. Osteoimmunology: Interplay between the immune system and bone metabolism. Annu. Rev. Immunol. 2006, 24, 33–63. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hayashi, M.; Takayanagi, H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol. Metab. 2012, 23, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-κB-mediated regulation of osteoclastogenesis. Endocrinol. Metab. 2015, 30, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Negishi-Koga, T.; Takayanagi, H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol. Rev. 2009, 231, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Galson, D.L.; Zhao, C.; Peng, L.; Laplace, C.; Wang, K.Z.; Bachler, M.A.; Amano, H.; Aburatani, H.; Ishikawa, H.; et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 2004, 279, 26475–26480. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Kang, J.W.; Lee, E.M.; Kim, J.S.; Rhee, Y.H.; Kim, M.; Jeong, S.J.; Park, Y.G.; Kim, S.H. Melatonin promotes osteoblastic differentiation through the BMP/ERK/Wnt signaling pathways. J. Pineal Res. 2011, 51, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Radio, N.M.; Kotlarczyk, M.P.; Chen, C.T.; Wei, Y.H.; Jockers, R.; Witt-Enderby, P.A. Determination of the minimal melatonin exposure required to induce osteoblast differentiation from human mesenchymal stem cells and these effects on downstream signaling pathways. J. Pineal Res. 2010, 49, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.; Witt-Enderby, P.A. Melatonin effects on bone: potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J. Pineal Res. 2014, 56, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Nakade, O; Takada, Y.; Kaku, T.; Lau, K.H. Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down-regulation of the RANKL-mediated osteoclast formation and activation. J. Bone Miner. Res. 2002, 17, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Amstrup, A.K.; Sikjaer, T.; Mosekilde, L.; Rejnmark, L. Melatonin and the skeleton. Osteoporos. Int. 2013, 24, 2919–2927. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Choi, Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front. Immunol. 2014, 5, 511. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Lopez, L.; Perez-Beltran, C.; Ramirez-Rodriguez, G. Chronic administration of a melatonin membrane receptor antagonist, luzindole, affects hippocampal neurogenesis without changes in hopelessness-like behavior in adult mice. Neuropharmacology 2016, 103, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, Y.; Chang, E.J.; Kim, H.M.; Hong, S.P.; Lee, Z.H.; Ryu, J.; Kim, H.H. Suppression of osteoclastogenesis by N,N-dimethyl-d-erythro-sphingosine: A sphingosine kinase inhibition-independent action. Mol. Pharmacol. 2007, 72, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Takatsuna, H.; Asagiri, M.; Kubota, T.; Oka, K.; Osada, T.; Sugiyama, C.; Saito, H.; Aoki, K.; Ohya, K.; Takayanagi, H.; et al. Inhibition of RANKL-induced osteoclastogenesis by (−)-DHMEQ, a novel NF-κB inhibitor, through downregulation of NFATc1. J. Bone Miner. Res. 2005, 20, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Satue, M.; Ramis, J.M.; del Mar Arriero, M.; Monjo, M. A new role for 5-methoxytryptophol on bone cells function in vitro. J. Cell. Biochem. 2015, 116, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Somei, M.; Kitamura, K.; Reiter, R.J.; Hattori, A. Novel bromomelatonin derivatives suppress osteoclastic activity and increase osteoblastic activity: Implications for the treatment of bone diseases. J. Pineal Res. 2008, 44, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Zhdanova, I.V.; Wurtman, R.J.; Balcioglu, A.; Kartashov, A.I.; Lynch, H.J. Endogenous melatonin levels and the fate of exogenous melatonin: Age effects. J. Gerontol. A Biol. Sci. Med. Sci. 1998, 53, B293–B298. [Google Scholar] [CrossRef] [PubMed]
- Waldhauser, F.; Waldhauser, M.; Lieberman, H.R.; Deng, M.H.; Lynch, H.J.; Wurtman, R.J. Bioavailability of oral melatonin in humans. Neuroendocrinology 1984, 39, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Zhang, M.; Weintraub, S.T.; Cabrera, J.; Sainz, R.M.; Mayo, J.C. Identification of highly elevated levels of melatonin in bone marrow: Its origin and significance. Biochim. Biophys. Acta 1999, 1472, 206–214. [Google Scholar] [CrossRef]
- Hirotani, H.; Tuohy, N.A.; Woo, J.T.; Stern, P.H.; Clipstone, N.A. The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW264.7 cells. J. Biol. Chem. 2004, 279, 13984–13992. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Prasad, V.; Hyung, S.W.; Lee, Z.H.; Lee, S.W.; Bhargava, A.; Pearce, D.; Lee, Y.; Kim, H.H. Plasma membrane calcium ATPase regulates bone mass by fine-tuning osteoclast differentiation and survival. J. Cell Biol. 2012, 199, 1145–1158. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Kim, H.J.; Bae, M.-K.; Kim, Y.-D. Suppression of Osteoclastogenesis by Melatonin: A Melatonin Receptor-Independent Action. Int. J. Mol. Sci. 2017, 18, 1142. https://doi.org/10.3390/ijms18061142
Kim HJ, Kim HJ, Bae M-K, Kim Y-D. Suppression of Osteoclastogenesis by Melatonin: A Melatonin Receptor-Independent Action. International Journal of Molecular Sciences. 2017; 18(6):1142. https://doi.org/10.3390/ijms18061142
Chicago/Turabian StyleKim, Hyung Joon, Ha Jin Kim, Moon-Kyoung Bae, and Yong-Deok Kim. 2017. "Suppression of Osteoclastogenesis by Melatonin: A Melatonin Receptor-Independent Action" International Journal of Molecular Sciences 18, no. 6: 1142. https://doi.org/10.3390/ijms18061142
APA StyleKim, H. J., Kim, H. J., Bae, M. -K., & Kim, Y. -D. (2017). Suppression of Osteoclastogenesis by Melatonin: A Melatonin Receptor-Independent Action. International Journal of Molecular Sciences, 18(6), 1142. https://doi.org/10.3390/ijms18061142