Hepatoprotective Effect of Aqueous Extract from the Seeds of Orychophragmus violaceus against Liver Injury in Mice and HepG2 Cells
Abstract
:1. Introduction
2. Results
2.1. High Performance Liquid Chromatography (HPLC) Analysis and Composition of AEOV
2.2. Hepatoprotective Effect of AEOV against CCl4-Induced Liver Injury
2.3. Anti-Oxidative Effect of AEOV on Liver Tissues
2.4. Anti-Inflammatory Effect of AEOV on Liver Tissues
2.5. Cytoprotective Effect of Epigoitrin against H2O2-Induced HepG2 Cells
2.6. Anti-Oxidative and Anti-Inflammatory Effects of Epigoitrin against H2O2-Induced HepG2 Cells
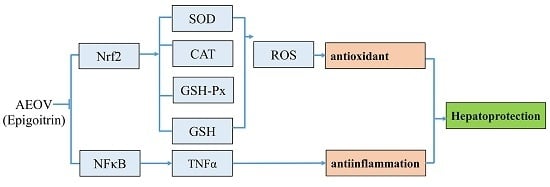
3. Discussion
4. Methods
4.1. Plant Material and Extract Preparation
4.2. HPLC Analysis
4.3. Cell Culture
4.4. Cell Viability Evaluation
4.5. Measurement of LDH, MDA, SOD, and GSH- Px in H2O2-Induced HepG2 Cells
4.6. Animals
4.7. Induction of CCl4-Mediated Liver Injury
4.8. Liver Weight and Liver Index
4.9. Serum Biochemistry
4.10. Assay of Reactive Oxygen Species (ROS) and Hepatic Enzymes in Liver Tissues
4.11. Histological Analysis
4.12. Immunohistochemical Analyses
4.13. Western Blot Analysis
4.14. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, Y.X.; Qiu, Y.Q.; Xu, L.Q.; Guo, J.; Li, L.J. Xiao-chai-hu tang in treating model mice with d-galactosamine-induced liver injury. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, M.; Qiu, B.; Cui, T.; Hamada, A.; Yoshioka, S.; Ono, M.; Miyamura, M.; Kyotani, S.; Nishioka, Y. Effect of sho-saiko-to extract on hepatic inflammation and fibrosis in dimethylnitrosamine induced liver injury rats. Biol. Pharm. Bull. 2002, 25, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Arteel, G.E. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology 2003, 124, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Cederbaum, A.I. Alcohol and oxidative liver injury. Hepatology 2006, 43, S63–S74. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Wang, H.; Park, O.; Horiguchi, N.; Lafdil, F.; Mukhopadhyay, P.; Moh, A.; Fu, X.Y.; Kunos, G.; Pacher, P.; et al. Anti-Inflammatory and anti-apoptotic roles of endothelial cell STAT3 in alcoholic liver injury. Alcohol. Clin. Exp. Res. 2010, 34, 719–725. [Google Scholar] [CrossRef] [PubMed]
- He, Y.M.; Zhu, S.; Ge, Y.W.; Kazuma, K.; Zou, K.; Cai, S.Q.; Komatsu, K. The anti-inflammatory secoiridoid glycosides from Gentianae Scabrae Radix: The root and rhizome of Gentiana scabra. J. Nat. Med. 2015, 69, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.T.; Jeong, S.C.; Hwang, J.S.; Kim, J.H. Modulation effects of sweroside isolated from the Lonicera japonica on melanin synthesis. Chem. Biol. Interact. 2015, 238, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.J.; Takashima, M.; Rippe, R.A. Molecular mechanisms of hepatic fibrogenesis. J. Gastroenterol. Hepatol. 2007, 22 (Suppl. S1), S79–S84. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.M.; Wang, Y.; Tan, H.S.; Yu, T.; Fan, X.M.; Chen, P.; Zeng, H.; Huang, M.; Bi, H.C. Schisandrol B protects against acetaminophen-induced acute hepatotoxicity in mice via activation of the Nrf2/ARE signaling pathway. Acta Pharmacol. Sin. 2016, 37, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Dodd, G.; Thomas, S.; Zhang, X.; Wasserman, M.A.; Rovin, B.H.; Kunsch, C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1862–H1870. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Itoh, K.; Nagayoshi, E.; Haruta, J.; Kimura, T.; O'Connor, T.; Harada, T.; Yamamoto, M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of are-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001, 59, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Kodali, P.; Wu, P.; Lahiji, P.A.; Brown, E.J.; Maher, J.J. Anit toxicity toward mouse hepatocytes in vivo is mediated primarily by neutrophils via CD18. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G355–G363. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.L.; Yang, F.; Gong, J.T.; Tang, X.W.; Wang, G.Y.; Wang, Z.T.; Yang, L. Sweroside ameliorates α-naphthylisothiocyanate-induced cholestatic liver injury in mice by regulating bile acids and suppressing pro-inflammatory responses. Acta Pharmacol. Sin. 2016, 37, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Heathcote, E.J.; Gershwin, M.E. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology 2010, 139, 1481–1496. [Google Scholar] [CrossRef] [PubMed]
- Rahim, S.M.; Taha, E.M.; Al-janabi, M.S.; Al-douri, B.I.; Simon, K.D.; Mazlan, A.G. Hepatoprotective effect of cymbopogon citratus aqueous extract against hydrogen peroxide-induced liver injury in male rats. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.W.; Liu, M.; Li, Z.Y. Parental genome separation and elimination of cells and chromosomes revealed by AFLP and GISH analyses in a Brassica carinata × Orychophragmus violaceus cross. Ann. Bot. 2006, 97, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.W.; Xu, Z.Q.; Guo, X.H.; Li, R.J.; Shen, J.G.; Xu, X.D.; Zhang, B.X. Protective effect of aqueous extract from the seeds of Orychophragmus violaceus against acute liver injury induced by Cortex dictamni in mice. Chin. J. Pharmacol. Toxicol. 2016, 30, 101–106. [Google Scholar]
- Pingali, S.; Donahue, J.P.; Payton-Stewart, F. Tetrahydroberberine, a pharmacologically active naturally occurring alkaloid. Acta Crystallogr. C Struct. Chem. 2015, 71, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Huang, H.; Chen, J.; Li, X. In vitro antioxidant and anti-inflammatory activities of Radix isatidis extract and bioaccessibility of six bioactive compounds after simulated gastro-intestinal digestion. J. Ethnopharmacol. 2014, 157, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Santangelo, C.; D'Archivio, M.; Li Volti, G.; Giovannini, C.; Galvano, F. Protocatechuic acid and human disease prevention: Biological activities and molecular mechanisms. Curr. Med. Chem. 2012, 19, 2901–2917. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, C.; Scazzocchio, B.; Matarrese, P.; Vari, R.; D’Archivio, M.; Di Benedetto, R.; Casciani, S.; Dessi, M.R.; Straface, E.; Malorni, W.; et al. Apoptosis induced by oxidized lipids is associated with up-regulation of p66Shc in intestinal Caco-2 cells: Protective effects of phenolic compounds. J. Nutr. Biochem. 2008, 19, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Ye, W.; Chen, J.; Li, X. Antiviral activities against influenza virus (FM1) of bioactive fractions and representative compounds extracted from Banlangen (Radix isatidis). J. Tradit. Chin. Med. 2016, 36, 369–376. [Google Scholar] [PubMed]
- Park, C.H.; Tanaka, T.; Cho, E.J.; Park, J.C.; Shibahara, N.; Yokozawa, T. Glycerol-induced renal damage improved by 7-O-galloyl-d-sedoheptulose treatment through attenuating oxidative stress. Biol. Pharm. Bull. 2012, 35, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Liby, K.T. Cancer chemoprevention: Scientific promise, clinical uncertainty. Nat. Clin. Pract. Oncol. 2005, 2, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ravula, R.; Wang, Z.; Zuo, Z.; Chow, M.S.; Thakkar, A.; Prabhu, S.; Andresen, B.; Huang, Y. Traditional chinese medicinal formula si-wu-tang prevents oxidative damage by activating Nrf2-mediated detoxifying/antioxidant genes. Cell. Biosci. 2014, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Doh, S.T.; Chan, J.Y.; Kong, A.N.; Cai, L. Regulatory potential for concerted modulation of Nrf2- and NFκB1-mediated gene expression in inflammation and carcinogenesis. Br. J. Cancer 2008, 99, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamamoto, M. Molecular mechanisms activating the Nrf2-keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 2005, 7, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, K.R.; Yan, X.X.; Freeman, M.L. Nrf2 degradation by the ubiquitin proteasome pathway is inhibited by KIAA0132, the human homolog to INrf2. Oncogene 2002, 21, 6829–6834. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yan, W.; Chen, S.; Sun, C.R.; Zhang, J.M. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta Pharmacol. Sin. 2010, 31, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Kaspar, J.W.; Shen, J.; Jaiswal, A.K. Nrf2 signaling and cell survival. Toxicol. Appl. Pharmacol. 2010, 244, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamamoto, M. Nrf2-keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.D.; Carlson, B.A.; Li, F.; Bonzo, J.A.; Yoo, M.H.; Krausz, K.W.; Conrad, M.; Chen, C.; Gonzalez, F.J.; Hatfield, D.L. Disruption of thioredoxin reductase 1 protects mice from acute acetaminophen-induced hepatotoxicity through enhanced Nrf2 activity. Chem. Res. Toxicol. 2013, 26, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Goldring, C.E.; Kitteringham, N.R.; Elsby, R.; Randle, L.E.; Clement, Y.N.; Williams, D.P.; McMahon, M.; Hayes, J.D.; Itoh, K.; Yamamoto, M.; et al. Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology 2004, 39, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Kansanen, E.; Kivela, A.M.; Levonen, A.L. Regulation of Nrf2-dependent gene expression by 15-deoxy-δ12,14-prostaglandin J2. Free Radic. Biol. Med. 2009, 47, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Han, X.D.; Kan, Y.W. An important function of Nrf2 in combating oxidative stress: Detoxification of acetaminophen. Proc. Natl. Acad. Sci. USA 2001, 98, 4611–4616. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Jean, P.A.; Roth, R.A. Bile duct epithelial cells exposed to α-naphthylisothiocyanate produce a factor that causes neutrophil-dependent hepatocellular injury in vitro. Toxicol. Sci. 1999, 47, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, Z.X.; Sun, L.X.; Li, X.; Xu, Z.M.; Chen, M.; Zhao, G.L.; Jiang, Z.Z.; Zhang, L.Y. Resveratrol effectively attenuates α-naphthyl-isothiocyanate-induced acute cholestasis and liver injury through choleretic and anti-inflammatory mechanisms. Acta Pharmacol. Sin. 2014, 35, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Dahm, L.J.; Schultze, A.E.; Roth, R.A. An antibody to neutrophils attenuates α-naphthylisothiocyanate-induced liver injury. J. Pharmacol. Exp. Ther. 1991, 256, 412–420. [Google Scholar] [PubMed]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Scholmerich, J.; Gross, V. Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998, 115, 357–369. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear factor-κB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, L.; Gao, L.; Guo, Y.; Zhang, L.; Li, L.; Si, J.; Cao, L. Antiinflammatory and analgesic activities of ethanol extract and isolated compounds from Millettia pulchra. Biol. Pharm. Bull. 2015, 38, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Bolati, D.; Shimizu, H.; Yisireyili, M.; Nishijima, F.; Niwa, T. Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-κB. BMC Nephrol. 2013, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Bolati, D.; Higashiyama, Y.; Nishijima, F.; Shimizu, K.; Niwa, T. Indoxyl sulfate upregulates renal expression of MCP-1 via production of ROS and activation of NF-κB, p53, ERK, and JNK in proximal tubular cells. Life Sci. 2012, 90, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.T.; Strack, P.; Beer-Romero, P.; Chu, C.Y.; Elledge, S.J.; Harper, J.W. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 1999, 13, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Horii, S.; Matsuzaki, Y.; Ono, S.; Shimmura, Y.; Sato, K.; Egashira, Y. Anti-inflammatory effect of pyroglutamyl-leucine on lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2014, 117, 1–6. [Google Scholar] [CrossRef] [PubMed]








| Group | Dose (mg/kg) | Body Weight (g) | Relative Liver Weight (g/100 g Body Weight) |
|---|---|---|---|
| Ι | - | 19.7 ± 0.89 | 4.67 ± 0.17 |
| II | - | 20.13 ± 0.6 | 5.02 ± 0.22 |
| ΙΙΙ | 125 | 19.21 ± 0.91 | 4.65 ± 0.39 |
| ΙV | 250 | 19.68 ± 0.65 | 4.85 ± 0.16 |
| V | 500 | 19.85 ± 0.76 | 4.84 ± 0.22 |
| VI | 150 | 20.6 ± 0.8 | 5.13 ± 0.21 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, X.; Liu, C.; Gao, L.; Xu, X.; Zhu, N.; Cao, L. Hepatoprotective Effect of Aqueous Extract from the Seeds of Orychophragmus violaceus against Liver Injury in Mice and HepG2 Cells. Int. J. Mol. Sci. 2017, 18, 1197. https://doi.org/10.3390/ijms18061197
Huo X, Liu C, Gao L, Xu X, Zhu N, Cao L. Hepatoprotective Effect of Aqueous Extract from the Seeds of Orychophragmus violaceus against Liver Injury in Mice and HepG2 Cells. International Journal of Molecular Sciences. 2017; 18(6):1197. https://doi.org/10.3390/ijms18061197
Chicago/Turabian StyleHuo, Xiaowei, Chenqi Liu, Li Gao, Xudong Xu, Nailiang Zhu, and Li Cao. 2017. "Hepatoprotective Effect of Aqueous Extract from the Seeds of Orychophragmus violaceus against Liver Injury in Mice and HepG2 Cells" International Journal of Molecular Sciences 18, no. 6: 1197. https://doi.org/10.3390/ijms18061197
APA StyleHuo, X., Liu, C., Gao, L., Xu, X., Zhu, N., & Cao, L. (2017). Hepatoprotective Effect of Aqueous Extract from the Seeds of Orychophragmus violaceus against Liver Injury in Mice and HepG2 Cells. International Journal of Molecular Sciences, 18(6), 1197. https://doi.org/10.3390/ijms18061197