Biocompatibility of Four Common Orthopedic Biomaterials Following a High-Salt Diet: An In Vivo Study
Abstract
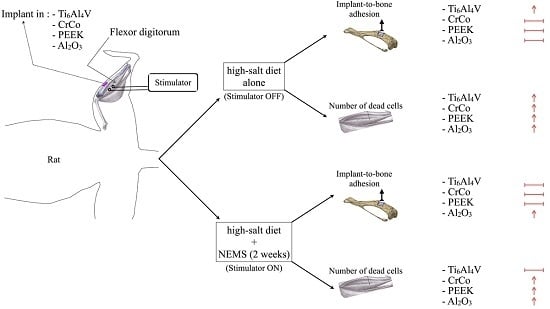
:1. Introduction
2. Results
2.1. Implant-To-Bone Adhesion
2.2. Muscle Damage
3. Discussion
3.1. Implant-To-Bone Adhesion
3.2. Number of Dead Cells
3.3. Consideration in Regard to Normalized Value
4. Materials and Methods
4.1. Models
4.2. Ethical Approval
4.3. Implant Design
4.4. Surgical Protocol
4.5. High-Salt Diet
4.6. NMES Program
4.7. Muscle and Bone Sampling
4.8. Implant-To-Bone Adhesion
4.9. Muscle Cell Death Analysis
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech. Rep. Ser. 2003, 1–149.
- Franco, V.; Oparil, S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J. Am. Coll. Nutr. 2006, 25, 247s–255s. [Google Scholar] [CrossRef] [PubMed]
- James, W.P.T.; Ralph, A.; Sanchezcastillo, C.P. The dominance of salt in manufactured food in the sodium-intake of affluent societies. Lancet 1987, 1, 426–429. [Google Scholar] [CrossRef]
- Engstrom, A.; Tobelmann, R.C.; Albertson, A.M. Sodium intake trends and food choices. Am. J. Clin. Nutr. 1997, 65, 704–707. [Google Scholar]
- MacGregor, G.A. Salt—More adverse effects. Am. J. Hypertens. 1997, 10, S37–S41. [Google Scholar] [CrossRef]
- Frassetto, L.A.; Morris, R.C.; Sellmeyer, D.E.; Sebastian, A. Adverse effects of sodium chloride on bone in the aging human population resulting from habitual consumption of typical American diets. J. Nutr. 2008, 138, 419s–422s. [Google Scholar] [PubMed]
- Sarmugam, R.; Worsley, A. Current levels of salt knowledge: A review of the literature. Nutrients 2014, 6, 5534–5559. [Google Scholar] [CrossRef] [PubMed]
- Buehlmeier, J.; Frings-Meuthen, P.; Remer, T.; Maser-Gluth, C.; Stehle, P.; Biolo, G.; Heer, M. Alkaline salts to counteract bone resorption and protein wasting induced by high salt intake: Results of a randomized controlled trial. J. Clin. Endocrinol. Metabol. 2012, 97, 4789–4797. [Google Scholar] [CrossRef] [PubMed]
- Frings-Meuthen, P.; Baecker, N.; Heer, M. Low-grade metabolic acidosis may be the cause of sodium chloride-induced exaggerated bone resorption. J. Bone Miner. Res. 2008, 23, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Frings-Meuthen, P.; Buehlmeier, J.; Baecker, N.; Heer, M. Effects of potassium bicarbonate on calcium- and bone metabolism during high salt intake. J. Bone Miner. Res. 2008, 23, S217. [Google Scholar]
- Massey, L.K.; Whiting, S.J. Dietary salt, urinary calcium, and bone loss. J. Bone Miner. Res. 1996, 11, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Creedon, A.; Cashman, K.D. The effect of high salt and high protein intake on calcium metabolism, bone composition and bone resorption in the rat. Br. J. Nutr. 2000, 84, 49–56. [Google Scholar] [PubMed]
- Creedon, A.; Flynn, A.; Cashman, K.D. The effect of dietary sodium and protein intakes on bone composition and bone resorption in the rat. Proc. Nutr. Soc. 2000, 59, 89a. [Google Scholar]
- Teucher, B.; Fairweather-Tait, S. Dietary sodium as a risk factor for osteoporosis: Where is the evidence? Proc. Nutr. Soc. 2003, 62, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Role of dietary sodium in osteoporosis. J. Am. Coll. Nutr. 2006, 25, 271s–276s. [Google Scholar] [CrossRef] [PubMed]
- Stompor, T.; Zablocki, M.; Lesiow, M. Osteoporosis in mineral and bone disorders of chronic kidney disease. Pol. Arch. Med. Wewn. 2013, 123, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Dixon, T.; Shaw, M.; Ebrahim, S.; Dieppe, P. Trends in hip and knee joint replacement: Socioeconomic inequalities and projections of need. Ann. Rheum. Dis. 2004, 63, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Haleem-Smith, H.; Argintar, E.; Bush, C.; Hampton, D.; Postma, W.F.; Chen, F.H.; Rimington, T.; Lamb, J.; Tuan, R.S. Biological responses of human mesenchymal stem cells to titanium wear debris particles. J. Orthop. Res. 2012, 30, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Raska, M. Particle disease: Biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013, 19, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Wimmer, M.A.; Holinka, M. Osteolysis around total knee arthroplasty: A review of pathogenetic mechanisms. Acta Biomater. 2013, 9, 8046–8058. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J. Biologic responses to orthopedic implants innate and adaptive immune responses to implant debris. Spine 2016, 41, A30–A31. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Jacobs, J.J. Biologic effects of implant debris. Bull. NYU Hosp. Jt. Dis. 2009, 67, 182–188. [Google Scholar] [PubMed]
- Papakyriacou, M.; Mayer, H.; Pypen, C.; Plenk, H.; Stanzl-Tschegg, S. Influence of loading frequency on high cycle fatigue properties of b.c.c. and h.c.p. metals. Mater. Sci. Eng. A 2001, 308, 143–152. [Google Scholar] [CrossRef]
- Lewis, A.C.; Kilburn, M.R.; Papageorgiou, I.; Allen, G.C.; Case, C.P. Effect of synovial fluid, phosphate-buffered saline solution, and water on the dissolution and corrosion properties of CoCrMo alloys as used in orthopedic implants. J. Biomed. Mater. Res. A 2005, 73A, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, Y.; Wallinder, I.O. Metal release and speciation of released chromium from a biomedical CoCrMo alloy into simulated physiologically relevant solutions. J. Biomed. Mater. Res. B 2014, 102, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J. Effect of particulate cobalt, chromium and cobalt-chromium alloy on human osteoblast-like cells in vitro—Reply. J. Bone Jt. Surg. 1998, 80B, 934. [Google Scholar]
- Muralidharan, V.S. Role of anions in the dissolution, passivation and pitting of metals. A review. Corrosion Rev. 2003, 21, 327–347. [Google Scholar] [CrossRef]
- Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Oda, Y. Discoloration of titanium alloy in acidic saline solutions with peroxide. Dent. Mater. J. 2013, 32, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Suter, T.; Bohni, H. A new microelectrochemical method to study pit initiation on stainless steels. Electrochim. Acta 1997, 42, 3275–3280. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Electrical implications of corrosion for osseointegration of titanium implants. J. Dent. Res. 2011, 90, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, M.; Felix, M.S.; Linares, J.M.; Chaves-Jacob, J.; Decherchi, P.; Dousset, E. Titanium implant impairment and surrounding muscle cell death following high-salt diet: An in vivo study. PLoS ONE 2016, 11, e0146873. [Google Scholar] [CrossRef] [PubMed]
- Meghji, S.; Morrison, M.S.; Henderson, B.; Arnett, T.R. pH dependence of bone resorption: Mouse calvarial osteoclasts are activated by acidosis. Am. J. Physiol. Endocrinol. Metabol. 2001, 280, E112–E119. [Google Scholar]
- Krieger, N.S.; Sessler, N.E.; Bushinsky, D.A. Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am. J. Physiol. 1992, 262, F442–F448. [Google Scholar] [PubMed]
- Arnett, T.R.; Spowage, M. Modulation of the resorptive activity of rat osteoclasts by small changes in extracellular pH near the physiological range. Bone 1996, 18, 277–279. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Toth, J.M.; Wang, M.; Estes, B.T.; Scifert, J.L.; Seim, H.B.; Turner, A.S. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials 2006, 27, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. Polyetheretherketone for long-term implantable devices. Med. Device Technol. 2008, 19, 987–992. [Google Scholar]
- Hunter, A.; Archer, C.W.; Walker, P.S.; Blunn, G.W. Attachment and proliferation of osteoblasts and fibroblasts on biomaterials for orthopedic use. Biomaterials 1995, 16, 287–295. [Google Scholar] [CrossRef]
- Morrison, C.; Macnair, R.; Macdonald, C.; Wykman, A.; Goldie, I.; Grant, M.H. In Vitro biocompatibility testing of polymers for orthopedic implants using cultured fibroblasts and osteoblasts. Biomaterials 1995, 16, 987–992. [Google Scholar] [CrossRef]
- Wu, S.H.; Li, Y.; Zhang, Y.Q.; Li, X.K.; Yuan, C.F.; Hao, Y.L.; Zhang, Z.Y.; Guo, Z. Porous titanium-6 aluminum-4 vanadium cage has better osseointegration and less micromotion than a poly-ether-ether-ketone cage in sheep vertebral fusion. Artif. Organs 2013, 37, E191–E201. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Navarrete, R.; Gittens, R.A.; Schneider, J.M.; Hyzy, S.L.; Haithcock, D.A.; Ullrich, P.F.; Schwartz, Z.; Boyan, B.D. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012, 12, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, M.; Felix, M.S.; Bernard, C.; Linares, J.M.; Chaves-Jacob, J.; Decherchi, P.; Dousset, E. Biocompatibility of four common orthopedic biomaterials following neuro-electromyostimulation: An in vivo study. J. Biomed. Mater. Res. B 2017. [Google Scholar] [CrossRef]
- Hayashi, H.; Uchida, A.; Hamada, H.; Yoshikawa, H.; Shinto, Y.; Ono, K. Alumina ceramic prostheses for bone-tumor surgery. Arch. Orthop. Trauma Surg. 1992, 112, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, A.; Blessing, H.; Liedert, A.; Schmidt, C.; Neidlinger-Wilke, C.; Kaspar, D.; Friemert, B.; Claes, L. Tissue engineering of bone: Effects of mechanical strain on osteoblastic cells in type I collagen matrices. Biomaterials 2005, 26, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Toni, A.; Lewis, C.G.; Sudanese, A.; Stea, S.; Calista, F.; Savarino, L.; Pizzoferrato, A.; Giunti, A. Bone demineralization induced by cementless alumina-coated femoral stems. J. Arthrop. 1994, 9, 435–444. [Google Scholar] [CrossRef]
- Kubies, D.; Himmlova, L.; Riedel, T.; Chanova, E.; Balik, K.; Douderova, M.; Bartova, J.; Pesakova, V. The interaction of osteoblasts with bone-implant materials: 1. The effect of physicochemical surface properties of implant materials. Physiol. Res. 2011, 60, 95–111. [Google Scholar] [PubMed]
- Branemark, R.; Ohrnell, L.O.; Nilsson, P.; Thomsen, P. Biomechanical characterization of osseointegration during healing: An experimental in vivo study in the rat. Biomaterials 1997, 18, 969–978. [Google Scholar] [CrossRef]
- Frings-Meuthen, P.; Buehlmeier, J.; Baecker, N.; Stehle, P.; Fimmers, R.; May, F.; Kluge, G.; Heer, M. High sodium chloride intake exacerbates immobilization-induced bone resorption and protein losses. J. Appl. Physiol. 2011, 111, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.G.; Koh, H.S.; Kluess, D.; O’Connor, D.; Mathur, A.; Truskey, G.A.; Rubin, J.; Zhou, D.X.F.; Sung, K.L.P. Effects of titanium particle size on osteoblast functions in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 4578–4583. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Greene, A.S. Inhibition of angiogenesis by high salt diet is associated with impaired muscle performance following chronic muscle stimulation. Microcirculation 2008, 15, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Broderick, B.J.; Kennedy, C.; Breen, P.P.; Kearns, S.R.; O’Laighin, G. Patient tolerance of neuromuscular electrical stimulation (NMES) in the presence of orthopaedic implants. Med. Eng. Phys. 2011, 33, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Gotoh, E.; Manabe, T.; Kobayashi, K. Comparison of metal concentrations in rat tibia tissues with various metallic implants. Biomaterials 2004, 25, 5913–5920. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Williams, R.L.; Williams, D.F. The corrosion behaviour of Ti-6Al-4V, Ti-6Al-7Nb and Ti-13Nb-13Zr in protein solutions. Biomaterials 1999, 20, 631–637. [Google Scholar] [CrossRef]
- Khan, M.A.; Williams, R.L.; Williams, D.F. Conjoint corrosion and wear in titanium alloys. Biomaterials 1999, 20, 765–772. [Google Scholar] [CrossRef]
- Milosev, L.; Antolic, V.; Minovic, A.; Cor, A.; Herman, S.; Pavlovcic, V.; Campbell, P. Extensive metallosis and necrosis in failed prostheses with cemented titanium-alloy stems and ceramic heads. J. Bone Jt. Surg. 2000, 82B, 352–357. [Google Scholar] [CrossRef]
- Giants, T.W. Crystallinity and dielectric-properties of PEEK, poly (Ether Ether Ketone). IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 991–999. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Katz, B. The effect of sodium ions on the electrical activity of giant axon of the squid. J. Physiol. 1949, 108, 37–77. [Google Scholar] [CrossRef] [PubMed]
- Howie, D.W.; Rogers, S.D.; McGee, M.A.; Haynes, D.R. Biologic effects of cobalt chrome in cell and animal models. Clin. Orthop. Relat. Res. 1996, 329, S217–S232. [Google Scholar] [CrossRef]
- Granchi, D.; Cenni, E.; Trisolino, G.; Giunti, A.; Baldini, N. Sensitivity to implant materials in patients undergoing total hip replacement. J. Biomed. Mater. Res. B 2006, 77B, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.J.; Urban, R.M.; Wixson, R.L.; Meneghini, R.M.; Jacobs, J.J. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J. Bone Jt. Surg. 2013, 95A, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Tay, G.H.; Godbolt, D.B.; Crawford, R.W. Pseudotumor in a well-fixed metal-on-polyethylene uncemented hip arthroplasty. J. Arthrop. 2012, 493, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Endres, F.; El Abedin, S.Z.; Saad, A.Y.; Moustafa, E.M.; Borissenko, N.; Price, W.E.; Wallace, G.G.; MacFarlane, D.R.; Newman, P.J.; Bund, A. On the electrodeposition of titanium in ionic liquids. Phys. Chem. Chem. Phys. 2008, 10, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Endres, S.; Wilke, M.; Knoll, P.; Frank, H.; Kratz, M.; Wilke, A. Correlation of in vitro and in vivo results of vacuum plasma sprayed titanium implants with different surface topography. J. Mater. Sci. 2008, 19, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, M.; Felix, M.S.; Linares, J.M.; Chaves-Jacob, J.; Decherchi, P.; Dousset, E. Titanium implant impairment and surrounding muscle cell death following neuro-myoelectrostimulation: An in vivo study. J. Biomed. Mater. Res. B 2015, 103, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, A.O.; Mudashiru, S.K.; Alagbonsi, I.A. Effects of salt-loading hypertension on nociception in rats. J. Pain Res. 2013, 6, 387–392. [Google Scholar] [PubMed]
- Helle, F.; Karlsen, T.V.; Tenstad, O.; Titze, J.; Wiig, H. High-salt diet increases hormonal sensitivity in skin pre-capillary resistance vessels. Acta Physiol. 2013, 207, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Oloyo, A.K.; Nair, R.R.; Anigbogu, C.N.; Sofola, O.A. Relaxation response of abdominal aorta to androgens in orchidectomised Sprague-Dawley rats fed a high-salt diet. Can. J. Physiol. Pharmacol. 2012, 90, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Stevens-Lapsley, J.E.; Balter, J.E.; Wolfe, P.; Eckhoff, D.G.; Kohrt, W.M. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: A randomized controlled trial. Phys.Ther. 2012, 92, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Stevens-Lapsley, J.E.; Balter, J.E.; Wolfe, P.; Eckhoff, D.G.; Schwartz, R.S.; Schenkman, M.; Kohrt, W.M. Relationship between intensity of quadriceps muscle neuromuscular electrical stimulation and strength recovery after total knee arthroplasty. Phys. Ther. 2012, 92, 1187–1196. [Google Scholar] [CrossRef] [PubMed]






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecocq, M.; Bernard, C.; Felix, M.S.; Linares, J.-M.; Chaves-Jacob, J.; Decherchi, P.; Dousset, E. Biocompatibility of Four Common Orthopedic Biomaterials Following a High-Salt Diet: An In Vivo Study. Int. J. Mol. Sci. 2017, 18, 1489. https://doi.org/10.3390/ijms18071489
Lecocq M, Bernard C, Felix MS, Linares J-M, Chaves-Jacob J, Decherchi P, Dousset E. Biocompatibility of Four Common Orthopedic Biomaterials Following a High-Salt Diet: An In Vivo Study. International Journal of Molecular Sciences. 2017; 18(7):1489. https://doi.org/10.3390/ijms18071489
Chicago/Turabian StyleLecocq, Mathieu, Cécile Bernard, Marie Solenne Felix, Jean-Marc Linares, Julien Chaves-Jacob, Patrick Decherchi, and Erick Dousset. 2017. "Biocompatibility of Four Common Orthopedic Biomaterials Following a High-Salt Diet: An In Vivo Study" International Journal of Molecular Sciences 18, no. 7: 1489. https://doi.org/10.3390/ijms18071489
APA StyleLecocq, M., Bernard, C., Felix, M. S., Linares, J. -M., Chaves-Jacob, J., Decherchi, P., & Dousset, E. (2017). Biocompatibility of Four Common Orthopedic Biomaterials Following a High-Salt Diet: An In Vivo Study. International Journal of Molecular Sciences, 18(7), 1489. https://doi.org/10.3390/ijms18071489