Impact of the Hydration States of Polymers on Their Hemocompatibility for Medical Applications: A Review
Abstract
:1. Introduction
1.1. General Aspects of Water
1.2. Water in Biological Systems
1.3. Surface Water
1.4. Biocompatibility
“Biocompatibility refers to the ability of a biomaterial to perform its desired function with respect to a medical therapy, without eliciting any undesirable local or systemic effects in the recipient or beneficiary of that therapy, but generating the most beneficial cellular or tissue response in that specific situation, and optimizing the clinically relevant performance of that therapy.”
- Citotoxicity: cell damage caused by direct contact with the material or by leached compounds.
- Hemocompatibility: effect on blood or blood components such as breakdown of blood cells, immunologic response, and thrombus formation.
- Degradation: breakdown of the device, which products might cause toxicity.
- Implantation: local effects of the implant on tissue.
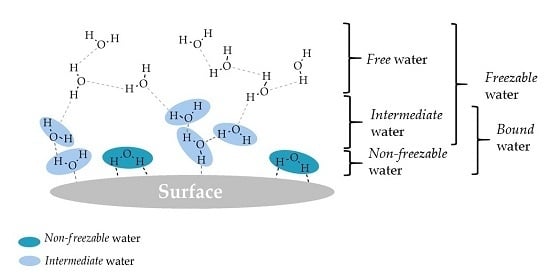
2. Types of Water
2.1. Denominations
- Non-freezable water is tightly bound to the surface and the water-surface interactions are very strong, while water–water interactions are very weak.
- Intermediate water interacts moderately with the surface (stronger than free but weaker than non-freezable water), involving both water-surface and water–water interactions.
- Free water hardly interacts with the surface and there is mainly water–water interaction.
2.2. Water Measurement Techniques
2.2.1. Differential Scanning Calorimetry
2.2.2. Nuclear Magnetic Resonance
- For non-freezable water the spectra is broad, showing low mobility due to strong interaction with the surface.
- For free water the spectra is narrow, very similar to bulk water, which means it has high mobility.
- For intermediate water the spectra is somewhere in between the spectra of the other two types of water, meaning that the mobility is intermediate.
2.2.3. Fourier Transform Infrared Spectroscopy
2.2.4. Other Techniques
3. Studies on the States of Water on Polymers and Their Effect on Biocompatibility
3.1. Degradable Polymers
3.1.1. l-Tyrosine Derived Polyarylates
3.1.2. Poly(ethylene glycol)
3.1.3. Aliphatic Carbonyls
3.1.4. Poly(lactic-co-glycolic) Acid
3.1.5. Poly(vinyl alcohol)
3.2. Non-Degradable Polymers
3.2.1. Poly(meth)acrylates
3.2.2. Poly(acrylonitrile)-co-N-2-vinyl-pyrrolidone
4. Discussion
4.1. Water States in Polymers
4.2. Biological Response in Polymers
4.3. Effect of Water States on Biological Response
5. Conclusions
Acknowledgments
Conflicts of Interest
Nomenclature
| Enthalpy change of melting of ice of bulk water | |
| Enthalpy change of melting of ice | |
| Enthalpy change of the cold-crystallization of ice | |
| %Free | Weight percentage of free water |
| %Int | Weight percentage of intermediate water |
| %NF | Weight percentage of non-freezable water |
| Calculated area of peak of NRM signal | |
| Measured area of peak of NRM signal | |
| Area of peak of NRM signal of pure water | |
| ATR | Attenuated total reflection |
| Cp | Specific heat capacity |
| DSC | Differential scanning calorimetry |
| DTB | Desaminotyrosyl-tyrosine butyl ester |
| DTBn | Desaminotyrosyl-tyrosine benzyl ester |
| DTE | Desaminotyrosyl-tyrosine ethyl ester |
| DTH | Desaminotyrosyl-tyrosine hexyl ester |
| DTiP | Desaminotyrosyl-tyrosine isopropyl ester |
| DTM | Desaminotyrosyl-tyrosine methyl ester |
| DTO | Desaminotyrosyl-tyrosine octyl ester |
| DTsb | Desaminotyrosyl-tyrosine sec-butyl ester |
| Equilibrium water content | |
| Fg | Fibrinogen |
| FT-IR | Fourier transform infrared |
| GA | Glycolic acid |
| HTE | 4-hydroxyphenylacetic acid-tyrosine ethyl ester |
| HTH | 4-hydroxyphenylacetic acid-tyrosine hexyl ester |
| IR | Infrared |
| LA | Lactic acid |
| Molecular weight per polymer repeating unit | |
| MTDSC | Modulated differential scanning calorimetry |
| Mν | Viscosity-average molecular weight |
| Molecular weight of water | |
| Mw | Molecular weight |
| NMR | Nuclear magnetic resonance |
| NVP | N-vinyl-2-pyrrolidone |
| Number of water molecules per polymer repeating unit | |
| Number of free water molecules per polymer repeating unit | |
| Number of non-freezable water molecules per polymer repeating unit | |
| PAN | Poly(acrylonitrile) |
| PANcNVP | Poly(acrylonitrile)-co-N-2-vinyl-pyrrolidone |
| PBA | Poly(n-butyl acrylate) |
| PCL | Poly(ε-caprolactone) |
| PDO | Poly(dioxanone) |
| PEA | Poly(ethyl acrylate) |
| Height of peak in NMR signal | |
| Width of the peak in NMR signal | |
| PEG | Poly(ethylene glycol) |
| PEHA | Poly(2-ethylhexyl acrylate) |
| PHEMA | Poly(2-hydroxyethyl methacrylate) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PMEA | Poly(2-methoxyethyl acrylate) |
| PMMA | Poly(methyl methacrylate) |
| PPEA | Poly(2-phenoxyethyl acrylate) |
| PRT | Plasma recalcification time |
| PTMC | Poly(trimethylene carbonate) |
| PVA | Poly(vinyl alcohol) |
| PVL | Poly(δ-valerolactone) |
| Heat associated to cold-crystallization process | |
| Heat associated to melting process | |
| SEM | Scanning electron microscope |
| Glass transition temperature | |
| TGA | Thermogravimetric analysis |
| Water content | |
| Weight of dry sample | |
| Mass of free water | |
| Weight percentage of freezable water | |
| Mass of intermediate water | |
| Mass of non-freezable water | |
| Weight percentage of non-freezable water | |
| Weight percentage of polymer | |
| Weight ratio of freezable water:polymer | |
| Weight ratio of non-freezable water:polymer | |
| Water uptake | |
| Weight of sorbed water | |
| Weight of wet sample | |
| XRD-DSC | X-ray diffraction with DSC |
References
- Robinson, G.W.; Singh, S.; Zhu, S-B.; Evans, M.W. Water in Biology, Chemistry, and Physics: Experimental Overviews and Computational Methodologies; World Scientific: Singapore, 1996. [Google Scholar]
- Ratner, B.D. Role of water in biomaterials. In Biomaterials Science: An Introduction to Materials in Medicine; Ratner, B.D., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 55–59. [Google Scholar]
- Chaplin, M.F. Water’s Hydrogen Bond Strength. In Water and Life: The Unique Properties of H2O; Lynden-Bell, R.M., Morris, S.C., Barrow, J.D., Finney, J.L., Harper, C., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 69–85. [Google Scholar]
- Bagchi, B. Water in Biological and Chemical Processes: From Structure and Dynamics to Function; Cambridge University Press: New York, NJ, USA, 2013; p. 5. [Google Scholar]
- Jhon, M.S.; Andrade, J.D. Water and hydrogels. J. Biomed. Mater. Res. 1973, 7, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Baier, R.E. Key Events in Blood Interactions at Nonphysiologic Interfaces—A Personal Primer. Artif. Organs 1978, 2, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Du, Z.; Yuan, S. Properties of a water layer on hydrophilic and hydrophobic self-assembled monolayer surfaces: A molecular dynamics study. Sci. China Chem. 2013, 56, 1–9. [Google Scholar] [CrossRef]
- Williams, D.F. Definitions in Biomaterials; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Williams, D.F. The Williams Dictionary of Biomaterials; Williams, D.F., Ed.; Liverpool University Press: Liverpool, UK, 1999. [Google Scholar]
- Williams, D.F. Concepts in biocompatibility: New biomaterials, new paradigms and new testing regimes. In Biocompatibility and Performance of Medical Devices; Boutrand, J.-P., Ed.; Woodhead Publising: Philadelphia, NJ, USA, 2012; pp. 9–12. [Google Scholar]
- Center for Devices and Radiological Health. Use of International Standard ISO 10993-1, “Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process”. Available online: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm348890.pdf (accessed on 15 December 2016).
- Xu, L.-C.; Bauer, J.; Siedlecki, C.A. Proteins, Platelets, and Blood Coagulation at Biomaterial Interfaces. Colloids Surf. B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Hechter, O.M.; Wittstruck, T.; McNiven, N.; Lester, G. Modification of the structure of water in agar gels. Proc. Natl. Acad. Sci. USA 1960, 46, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Sterling, C.; Masuzawa, M. Gel/water relationships in hydrophilic polymers: Nuclear magnetic resonance. Makromol. Chem. Phys. 1968, 116, 140–145. [Google Scholar] [CrossRef]
- McBrierty, V.J.; Martin, S.J.; Karasz, F.E. Understanding hydrated polymers: The perspective of NMR. J. Mol. Liq. 1999, 80, 179–205. [Google Scholar] [CrossRef]
- Higuchi, A.; Iijima, T. D.s.c. investigation of the states of water in poly(vinyl alcohol-co-itaconic acid) membranes. Polymer 1985, 26, 1833–1837. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T. Interaction between water and hydrophilic polymers. Thermochim. Acta 1998, 308, 3–22. [Google Scholar] [CrossRef]
- Tanaka, M.; Motomura, T.; Ishii, N.; Shimura, K.; Onishi, M.; Mochizuki, A.; Hatakeyama, T. Cold crystallization of water in hydrated poly(2-methoxyethyl acrylate) (PMEA). Polym. Int. 2000, 49, 1709–1713. [Google Scholar] [CrossRef]
- Hirata, Y.; Miura, Y.; Nakagawa, T. Oxygen permeability and the state of water in Nafion® membranes with alkali metal and amino sugar counterions. J. Memb. Sci. 1999, 163, 357–366. [Google Scholar] [CrossRef]
- Aizawa, M.; Suzuki, S. Properties of Water in Macromolecular Gels. III. Dilatometric Studies of the Properties of water in Macromolecular Gels. Bull. Chem. Soc. Jpn. 1971, 44, 2967–2971. [Google Scholar] [CrossRef]
- Tsuruta, T. On the role of water molecules in the interface between biological systems and polymers. J. Biomater. Sci. Polym. Ed. 2010, 21, 1831–1848. [Google Scholar] [CrossRef] [PubMed]
- Johari, G.; Hallbrucker, A.; Mayer, E. Two Calorimetrically Distinct States of Liquid Water Below 150 Kelvin. Science 1996, 273, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Qisui, W.; Xi, L.; Chaocan, Z. Investigation of the states of water and OH groups on the surface of silica. Colloids Surf. A Physicochem. Eng. Asp. 2009, 334, 112–115. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.; Yu, H. Effect of far-infrared drying on the water state and glass transition temperature in carrots. J. Food Eng. 2014, 136, 42–47. [Google Scholar] [CrossRef]
- Tylewicz, U.; Aganovic, K.; Vannini, M.; Toepfl, S.; Bortolotti, V.; Dalla Rosa, M.; Oey, I.; Heinz, V. Effect of pulsed electric field treatment on water distribution of freeze-dried apple tissue evaluated with DSC and TD-NMR techniques. Innov. Food Sci. Emerg. Technol. 2016, 37, 352–358. [Google Scholar] [CrossRef]
- Tanaka, M.; Hayashi, T.; Morita, S. The roles of water molecules at the biointerface of medical polymers. Polym. J. 2013, 45, 701–710. [Google Scholar] [CrossRef]
- Ping, Z.H.; Nguyen, Q.T.; Chen, S.M.; Zhou, J.Q.; Ding, Y.D. States of water in different hydrophilic polymers—DSC and FTIR studies. Polymer 2001, 42, 8461–8467. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Li, L.; Cheng, G.; Gong, X.; Zheng, J. Dual functionality of antimicrobial and antifouling of poly(n -hydroxyethylacrylamide)/salicylate hydrogels. J. Am. Chem. Soc. 2013, 128, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Fung, B.M.; McGaughy, T.W. The state of water in muscle as studied by pulsed NMR. Biochim. Biophys. Acta Gen. Subj. 1974, 343, 663–673. [Google Scholar] [CrossRef]
- MacRitchie, F. The liquid phase of dough and its role in baking. Cereal Chem. 1976, 53, 318–326. [Google Scholar]
- Schmitt, E.A.; Flanagan, D.R.; Linhardt, R.J. Importance of distinct water environments in the hydrolysis of poly(dl-lactide-co-glycolide). Macromolecules 1994, 27, 743–748. [Google Scholar] [CrossRef]
- Miwa, Y.; Ishida, H.; Tanaka, M.; Mochizuki, A. 2H-NMR and 13C-NMR study of the hydration behavior of poly(2-methoxyethyl acrylate), poly(2-hydroxyethyl methacrylate) and poly(tetrahydrofurfuryl acrylate) in relation to their blood compatibility as biomaterials. J. Biomater. Sci. Polym. Ed. 2010, 21, 1911–1924. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: West Sussex, UK, 2004; pp. 1–12. [Google Scholar]
- Morita, S.; Tanaka, M.; Ozaki, Y. Time-resolved in situ ATR-IR observations of the process of sorption of water into a poly(2-methoxyethyl acrylate) film. Langmuir 2007, 23, 3750–3761. [Google Scholar] [CrossRef] [PubMed]
- Kitano, H.; Mori, T.; Takeuchi, Y.; Tada, S.; Gemmei-Ide, M.; Yokoyama, Y.; Tanaka, M. Structure of water incorporated in sulfobetaine polymer films as studied by ATR-FTIR. Macromol. Biosci. 2005, 5, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Kishi, A.; Tanaka, M.; Mochizuki, A. Comparative study on water structures in polyHEMA and polyMEA by XRD-DSC simultaneous measurement. J. Appl. Polym. Sci. 2009, 111, 476–481. [Google Scholar] [CrossRef]
- Fiordeliso, J.; Bron, S.; Kohn, J. Design, synthesis, and pr eliminary characterization of tyrosine-containing polyarylates: New biomaterials for medical applications. J. Biomater. Sci. 1994, 5, 497–510. [Google Scholar] [CrossRef]
- Brocchini, S.; James, K.; Tangpasuthadol, V.; Kohn, J. A Combinatorial Approach for Polymer Design. J. Am. Chem. Soc. 1997, 119, 4553–4554. [Google Scholar] [CrossRef]
- Hooper, K.A.; Macon, N.D.; Kohn, J. Comparative histological evaluation of new tyrosine-derived polymers and poly (L-lactic acid) as a function of polymer degradation. J. Biomed. Mater. Res. 1998, 41, 443–454. [Google Scholar] [CrossRef]
- Lux Biosciences Gains Exclusive Worldwide License for Polyarylate Patent Estate From Rutgers University for Ophthalmic Use. Available online: http://www.prnewswire.com/news-releases/lux-biosciences-gains-exclusive-worldwide-license-for-polyarylate-patent-estate-from-rutgers-university-for-ophthalmic-use-56079057.html (accessed on 22 December 2016).
- FDA Approves First Medical Device Using Rutgers Biomaterial. Available online: https://www.eurekalert.org/pub_releases/2006-01/rtsu-faf010306.php (accessed on 22 December 2016).
- Kilfoyle, B.E.; Sheihet, L.; Zhang, Z.; Laohoo, M.; Kohn, J.; Michniak-Kohn, B.B. Development of paclitaxel-TyroSpheres for topical skin treatment. J. Control. Release 2012, 163, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, L.M.; Michniak, B.; Kohn, J. Variability of water uptake studies of biomedical polymers. J. Appl. Polym. Sci. 2011, 121, 1311–1320. [Google Scholar] [CrossRef]
- Weber, N.; Bolikal, D.; Bourke, S.L.; Kohn, J. Small changes in the polymer structure influence the adsorption behavior of fibrinogen on polymer surfaces: Validation of a new rapid screening technique. J. Biomed. Mater. Res. A 2004, 68, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, L.M.; Zhang, G.; Flach, C.R.; Murthy, N.S.; Mendelsohn, R.; Michniak-Kohn, B.; Kohn, J. Multiscale analysis of water uptake and erosion in biodegradable polyarylates. Polym. Degrad. Stab. 2012, 97, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, L.M.; Knight, D.D.; Kohn, J. Developing a Suitable Model for Water Uptake for Biodegradable Polymers Using Small Training Sets. Int. J. Biomater. 2016, 2016, 6273414. [Google Scholar] [CrossRef] [PubMed]
- Kohn, J.; Darr, A.; Schut, J. Polymers Derived from L-Tyrosine. In An Introduction to Biomaterials; Hollinger, J.O., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 309–315. [Google Scholar]
- Antonsen, K.P.; Hoffman, A.S. Water Structure of PEG Solutions by Differential Scanning Calorimetry Measurements. In Poly(Ethylene Glycol) Chemistry; Harris, J.M., Ed.; Plenum Press: New York, NY, USA, 1992; pp. 15–28. [Google Scholar]
- Harris, J.M. Introduction to Biotechnical and Biomedical Applications of Poly(Ethylene Glycol). In Poly(Ethylene Glycol) Chemistry: Biotechnical and Biomedical Applications; Harris, J.M., Ed.; Plenum Press: New York, NY, USA, 1992; pp. 1–14. [Google Scholar]
- Chen, Z.; Kang, L.; Wang, Z.; Xu, F.; Gu, G.; Cui, F.; Guo, Z. Recent progress in the research of biomaterials regulating cell behavior. RSC Adv. 2014, 4, 63807–63816. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chemie Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, X.-L.; Li, Z.-H.; Zhu, Z.-G.; Qian, S.-H.; Flewitt, A. Current and Emerging Technology for Continuous Glucose Monitoring. Sensors 2017, 17, 182. [Google Scholar] [CrossRef] [PubMed]
- Al-Halhouli, A.; Demming, S.; Alahmad, L.; Liobera, A.; Büttgenbach, S. An In-Line photonic biosensor for monitoring of glucose concentrations. Sensors 2014, 14, 15749–15759. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Polymer therapeutics: Top 10 selling pharmaceuticals—What next? J. Control. Release 2014, 190, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Tamai, N. A novel approach using differential scanning calorimetry to investigate the dissolved state in aqueous solutions of polymers used for papermaking. J. Appl. Polym. Sci. 2003, 89, 2798–2807. [Google Scholar] [CrossRef]
- Kitano, H.; Ichikawa, K.; Ide, I.; Fukuda, M.; Mizuno, W. Fourier transform infrared study on the state of water sorbed to poly(ethylene glycol) films. Langmuir 2001, 17, 1889–1895. [Google Scholar] [CrossRef]
- Nagaoka, S.; Mori, Y.; Takiuchi, H.; Yokota, K.; Tanzawa, H.; Nishiumi, S. Interaction between blood components and hydrogels with poly(oxyethylene) chains. In Polymers as Biomaterials; Shalaby, S.W., Hoffman, A.S., Ratner, B.D., Horbett, T.A., Eds.; Plenum Press: New York, NY, USA, 1985; pp. 361–374. [Google Scholar]
- Bergstrom, K.; Holmberg, K.; Safranj, A.; Hoffman, A.S.; Edgell, M.J.; Kozlowski, A.; Hovanes, B.A.; Harris, J.M. Reduction of fibrinogen adsorption on PEG-coated polystyrene surfaces. J. Biomed. Mater. Res. 1992, 26, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-T.; Cho, N.-S.; Yoon, H.-S.; Kim, T.-H.; Koh, M.-S.; Kim, W.-G. Biodegradable studies of poly(trimethylenecarbonate-ɛ-caprolactone)-block-poly(p-dioxanone), poly(dioxanone), and poly(glycolide-ɛ-caprolactone) (Monocryl®) monofilaments. J. Appl. Polym. Sci. 2006, 102, 737–743. [Google Scholar] [CrossRef]
- Wang, C.E.; Zhang, P.H. In vitro degradation behaviours of PDO monofilament and its intravascular stents with braided structure. Autex Res. J. 2016, 16, 80–89. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Rodriguez, I.A.; Wesner, D.; Schönherr, H.; Bowlin, G.L.; Jhurry, D. Poly(ester-ether)s: III. assessment of cell behaviour on nanofibrous scaffolds of PCL, PLLA and PDX blended with amorphous PMeDX. J. Mater. Chem. B 2015, 3, 673–687. [Google Scholar] [CrossRef]
- Fukushima, K.; Tsai, M.-Y.; Ota, T.; Haga, Y.; Matsuzaki, K.; Inoue, Y.; Tanaka, M. Evaluation of the hemocompatibility of hydrated biodegradable aliphatic carbonyl polymers with a subtle difference in the backbone structure based on the intermediate water concept and surface hydration. Polym. J. 2015, 47, 469–473. [Google Scholar] [CrossRef]
- Bartzoka, E.D.; Crestini, C.; Lange, H. Biomass Derived and Biomass Inspired Polymers in Pharmaceutical Applications. In Handbook of Polymers for Pharmaceutical Technologies, Bioactive and Compatible Synthetic/Hybrid Polymers; Thakur, V.K., Thakur, M.K., Eds.; Handbook of Polymers for Pharmaceutical Technologies; Scrivener Publishing LLC: Salem, MA, USA, 2015; pp. 127–204. [Google Scholar]
- Parikh, A.; Anand, U.; Ugwu, M.C.; Feridooni, T.; Massoud, E.; Agu, R.U. Drug-eluting nasal implants: Formulation, characterization, clinical applications and challenges. Pharmaceutics 2014, 6, 249–267. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K. Pasireotide in Acromegaly: A Review. Drugs 2015, 75, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Blasi, P.; D’Souza, S.S.; Selmin, F.; DeLuca, P.P. Plasticizing effect of water on poly(lactide-co-glycolide). J. Control. Release 2005, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Reading, M.; Luget, A.; Wilson, R. Modulated differential scanning calorimetry. Thermochim. Acta 1994, 238, 295–307. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Niu, X.; Zhou, G.; Li, P.; Fan, Y. Improved hemocompatibility and endothelialization of vascular grafts by covalent immobilization of sulfated silk fibroin on poly(lactic-co-glycolic acid) scaffolds. Biomacromolecules 2011, 12, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Muppalaneni, S. Polyvinyl Alcohol in Medicine and Pharmacy: A Perspective. J. Dev. Drugs 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Hodge, R.M.; Edward, G.H.; Simon, G.P. Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 1996, 37, 1371–1376. [Google Scholar] [CrossRef]
- Ino, J.M.; Sju, E.; Ollivier, V.; Yim, E.K. F.; Letourneur, D.; Le Visage, C. Evaluation of hemocompatibility and endothelialization of hybrid poly(vinyl alcohol) (PVA)/gelatin polymer films. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, N.; Ribeiro, J.; Gärtner, A.; Pereira, T.; Amorim, I.; Fragoso, J.; Lopes, A.; Fernandes, J.; Costa, E.; Santos-Silva, A.; et al. Biocompatibility and hemocompatibility of polyvinyl alcohol hydrogel used for vascular grafting-In vitro and in vivo studies. J. Biomed. Mater. Res. A 2014, 102, 4262–4275. [Google Scholar] [PubMed]
- Ellis, B.; Smith, R. Polymers: A Property Database, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 36–41. [Google Scholar]
- Oda, Y.; Horinouchi, A.; Kawaguchi, D.; Matsuno, H.; Kanaoka, S.; Aoshima, S.; Tanaka, K. Effect of side-chain carbonyl groups on the interface of vinyl polymers with water. Langmuir 2014, 30, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, F.; Deng, T.; Zhu, S.; Liu, W.; Zhong, H.; Yu, H.; Luo, R.; Deng, Z. Eudragit S100-Coated Chitosan Nanoparticles Co-loading Tat for Enhanced Oral Colon Absorption of Insulin. AAPS PharmSciTech 2017, 18, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Huanbutta, K.; Nernplod, T.; Akkaramongkolporn, P.; Sriamornsak, P. Design of porous Eudragit® L beads for floating drug delivery by wax removal technique. Asian J. Pharm. Sci. 2016, 12, 227–234. [Google Scholar] [CrossRef]
- Tanaka, M. Design of novel biointerfaces (I). Blood compatibility of poly(2-methoxyethyl acrylate). Biomed. Mater. Eng. 2004, 14, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Serkov, A.T.; Radishevskii, M.B. Status and prospects for production of carbon fibres based on polyacrylonitrile. Fibre Chem. 2008, 40, 24–31. [Google Scholar] [CrossRef]
- Wan, L.S.; Xu, Z.K.; Huang, X.J.; Wang, Z.G.; Ye, P. Hemocompatibility of poly(acrylonitrile-co-N-vinyl-2-pyrrolidone)s: Swelling behavior and water states. Macromol. Biosci. 2005, 5, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.-Q.; Xu, Z.-K.; Huang, X.-J.; Ye, P.; Wu, J. Acrylonitrile-Based Copolymer Membranes Containing Reactive Groups: Surface Modification by the Immobilization of Poly(ethylene glycol) for Improving Antifouling Property and Biocompatibility. Langmuir 2003, 19, 9889–9895. [Google Scholar] [CrossRef]
- Francois, P. Physical and biological effects of a surface coating procedure on polyurethane catheters. Biomaterials 1996, 17, 667–678. [Google Scholar] [CrossRef]
- Krasteva, N.; Harms, U.; Albrecht, W.; Seifert, B.; Hopp, M.; Altankov, G.; Groth, T. Membranes for biohybrid liver support systems—investigations on hepatocyte attachment, morphology and growth. Biomaterials 2002, 23, 2467–2478. [Google Scholar] [CrossRef]
- Staufenbiel, S.; Merino, M.; Li, W.; Huang, M.D.; Baudis, S.; Lendlein, A.; Müller, R.H.; Wischke, C. Surface characterization and protein interaction of a series of model poly[acrylonitrile-co-(N-vinyl pyrrolidone)] nanocarriers for drug targeting. Int. J. Pharm. 2015, 485, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.-S.; Huang, X.-J.; Xu, Z.-K. Diffusion and Structure of Water in Polymers Containing N-Vinyl-2-pyrrolidone. J. Phys. Chem. B 2007, 111, 922–928. [Google Scholar] [CrossRef] [PubMed]

























| Criteria | Types of Water | Reference | |
|---|---|---|---|
| Name | Criteria | ||
| Rigidity and mobility | Ice-like water a | Very low mobility | [13] |
| Intermediate between ice-like and free water b | Intermediate mobility | ||
| Free water c | High mobility | ||
| Solid water a (glass-like or ice-like) | Very low mobility | [14] | |
| Bound water b | Intermediate mobility | ||
| Free water c (very loosely bound or liquid) | High mobility | ||
| Tightly bound water a | Very low mobility at temperatures <230 K | [15] | |
| Loosely bound water b | Low mobility in the range 230–260 K | ||
| Free or bulk-like water c | Mobility similar to bulk water at around 273 K | ||
| Freezing temperature | Hydration water d | Freezes sub-zero or does not freeze | [19] |
| Free water e | Freezes at 0 °C | ||
| Bulk-like water | Normal mobility as normal melting point is approached | ||
| Non-freezing water or Non-freezing bound water f | No crystallization (no freezing) | [16,17,18] | |
| Freezing-bound water or Intermediate water g | Crystallization under 0 °C | ||
| Free water e | Normal crystallization of water | ||
| Thermal expansion | Hydrated water | Transition temperature: Non at −30 to 0 °C | [5,20] |
| Interfacial water | Transition temperature: −20 to 0 °C | ||
| “Normal” or bulk water | Transition temperature: 0 °C | ||
| Polymer | Max (wt %) | Fg Adsorption (% of Control) | Group | ||
|---|---|---|---|---|---|
| poly(DTO succinate) | 0.14 | 0.77 | 4 | 121.97 | Low |
| poly(DTB succinate) | 0.28 | 0.76 | 4 | 129.36 | |
| poly(HTE adipate) | 0.82 | 1.31 | 8 | 125.19 | |
| poly(DTO adipate) | 1.08 | 0.8 | 6 | 78.30 | |
| poly(DTM adipate) | 1.92 | 1.77 | 13 | 142.69 | |
| poly(DTM sebacate) | 1.98 | 1.03 | 12 | 99.14 | |
| poly(DTH suberate) | 2.25 | 1.03 | 10 | 91.68 | |
| poly(DTB adipate) | 3.45 | 1.76 | 19 | 127.12 | |
| poly(DTB glutarate) | 3.95 | 1.59 | 19 | 123.38 | |
| poly(HTH adipate) | 4.13 | 1.55 | 16 | 76.20 | |
| poly(DTE glutarate) | 4.13 | 1.91 | 22 | 151.44 | |
| poly(DTH adipate) | 4.26 | 2.01 | 18 | 82.27 | |
| poly(DTiP adipate) | 5.39 | 1.87 | 22 | 121.76 | |
| poly(DTE adipate) | 5.45 | 4.26 | 27 | 131.21 | |
| poly(DTBn adipate) | 6.98 | 2.40 | 30 | 142.16 | |
| poly(HTE succinate) | 9.14 | 3.27 | 30 | 182.15 | |
| poly(DTBn methyl adipate | 9.77 | 2.30 | 31 | 138.98 | |
| poly(DTBn suberate) | 15.71 | 2.26 | 47 | 92.10 | High |
| poly(DTM (R)(+) methyl adipate) | 23.67 | 3.51 | 56 | 125.70 | |
| poly(DTsB glutarate) | 23.70 | 1.80 | 49 | 132.32 | |
| poly(DTsB (R)(+) methyladipate) | 36.98 | 1.59 | 58 | 153.27 |
| Content of NVP (wt %) | Total Water (wt %) | Non-Freezable Water (wt %) | Freezable Water (wt %) |
|---|---|---|---|
| 0 | 29.7 | 4.6 | 25.1 |
| 7 | 30.6 | 5.2 | 25.4 |
| 15 | 43.3 | 9.6 | 33.7 |
| 22 | 55.5 | 16.0 | 39.5 |
| 31 | 58.3 | 19.3 | 39.0 |
| Polymer | Types of Water Measured | Biological Response Measured | Observations | References | Conclusions |
|---|---|---|---|---|---|
| PEG | Free, intermediate and non-freezable | Intermediate water is negligible at low Mw and increases with Mw until a constant value | [48,55] | Presence of intermediate water means low protein adsorption and platelet adhesion | |
| Platelet adhesion & plasma protein adsorption | Low protein adsorption and platelet adhesion | [48,57] | |||
| Aliphatic carbonyls | Free, intermediate and non-freezable | Platelet adhesion | There is lower platelet adhesion when intermediate water is present. | [62] | Presence of intermediate water means low platelet adhesion [62] |
| Poly(meth)acrylates | Free, intermediate and non-freezable | Platelet adhesion | Intermediate water present only in PMEA is responsible for its excellent hemocompatibility. | [18] | Presence of intermediate water means low platelet adhesion [18] |
| PLGA | Free, intermediate and non-freezable | No presence of intermediate water | [66] | Absence of intermediate water means high platelet adhesion. | |
| Fibrinogen adsorption & platelet adhesion | High fibrinogen adsorption and platelet adhesion | [44,68] | |||
| Cell attachment, morphology, viability; transcription level of genes and expression of proteins | PLGA with silk-fibroin has better biocompatibility | [68] | Presence of carbonyl group of fibroin allows intermediate water formation and better biocompatibility | ||
| PVA | Free, intermediate and non-freezable | PVA films have low intermediate water content | [70] | Low intermediate water content means high platelet adhesion (inactive state) | |
| Platelet adhesion | High platelet adhesion but in inactive state. | [71] | |||
| PANcNVP | Free, intermediate and non-freezable | Three types of water present Higher NVP means higher non-freezable water content | [79] [84] | High content of non-freezable water means less platelet adhesion [84] Intermediate water could influence hemocompatibility (not enough data for conclusions) | |
| Platelet adhesion & PRT | Higher amounts of NVP led to less platelet adhesion and increase of PRT | [79] | |||
| Polyarylates | Freezable and non-freezable | In polymers with over 10%, non-freezable water reaches a threshold lower than freezable water. | [43] | At high , high freezable water means high fibrinogen adsorption Intermediate water (implicit in freezable water) could influence hemocompatibility (not enough data for conclusions) | |
| Fibrinogen adsorption | Polymers with longer ester and diacid chains adsorb less fibrinogen | [44] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bag, M.A.; Valenzuela, L.M. Impact of the Hydration States of Polymers on Their Hemocompatibility for Medical Applications: A Review. Int. J. Mol. Sci. 2017, 18, 1422. https://doi.org/10.3390/ijms18081422
Bag MA, Valenzuela LM. Impact of the Hydration States of Polymers on Their Hemocompatibility for Medical Applications: A Review. International Journal of Molecular Sciences. 2017; 18(8):1422. https://doi.org/10.3390/ijms18081422
Chicago/Turabian StyleBag, Min A., and Loreto M. Valenzuela. 2017. "Impact of the Hydration States of Polymers on Their Hemocompatibility for Medical Applications: A Review" International Journal of Molecular Sciences 18, no. 8: 1422. https://doi.org/10.3390/ijms18081422
APA StyleBag, M. A., & Valenzuela, L. M. (2017). Impact of the Hydration States of Polymers on Their Hemocompatibility for Medical Applications: A Review. International Journal of Molecular Sciences, 18(8), 1422. https://doi.org/10.3390/ijms18081422