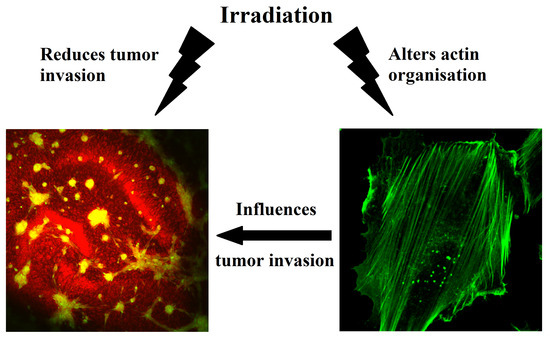
The Impact of Non-Lethal Single-Dose Radiation on Tumor Invasion and Cytoskeletal Properties
Abstract
:1. Introduction
2. Results
2.1. Impact of Radiation on Cell Proliferation and Cell Death
2.2. Analysis of Mechanical Properties by Atomic Force Microscopy
2.3. Analysis of Motile Properties Using Time Lapse Imaging
2.4. Tumor Invasion Measurements
2.5. Network Analysis of Single Cell Properties and Composite Parameters
2.6. Analysis of Actin Cytoskeleton Organization in Adherent Cells
3. Discussion
3.1. Effects of 2 Gy Single-Dose Radiation on Cell Proliferation and Survival
3.2. Effects of 2 Gy Single-Dose Radiation on Single Cell Properties
3.3. Effects of 2 Gy Single-Dose Radiation on Tumor Invasiveness, the Composite Parameter Stiffness, and the Actin Cytoskeleton
4. Materials and Methods
4.1. Cell Culture and Irradiation
4.2. Immunofluorescence and Immunohistochemical Staining
4.3. Western Blotting
4.4. Time Lapse Microscopy
4.5. Atomic Force Microscopy
4.6. Organotypic Hippocampal Slice Cultures (OHSC) and Tumor Invasion
4.7. Confocal Laser Scanning Microscopy (CLSM)
4.8. Network Analytical Approach
4.9. Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscope |
| CFDA | Fluorophores carboxyfluorescin diacetate |
| CTL | Control |
| EGFR | Epidermal growth factor receptor |
| FBS | Fetal bovine serum |
| GBM | Glioblastoma multiforme |
| OHSC | Organotypic hippocampal slice culture |
| PBS | Phosphate buffered saline |
| PCNA | Proliferating cell nuclear antigen |
| PI | Propidium iodide |
| P/S | Penicillin/streptomycin |
| sem | Standard error of the mean |
References
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, 2011–2030. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Green, S.B.; Byar, D.P.; Alexander, E.; Batzdorf, U.; Brooks, W.H.; Hunt, W.E.; MacCarty, C.S.; Mahaley, M.S.; Mealey, J.; et al. Randomized Comparisons of Radiotherapy and Nitrosoureas for the Treatment of Malignant Glioma after Surgery. N. Engl. J. Med. 1980, 303, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Alexander, E.; Hunt, W.E.; MacCarty, C.S.; Mahaley, M.S.; Mealey, J.; Norrell, H.A.; Owens, G.; Ransohoff, J.; Wilson, C.B.; et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: A cooperative clinical trial. J. Neurosurg. 1978, 49, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.G.; McCarthy, B.J.; Freels, S.; Kupelian, V.; Bondy, M.L. The conditional probability of survival of patients with primary malignant brain tumors: Surveillance, epidemiology, and end results (SEER) data. Cancer 1999, 85, 485–491. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. Cancer Radiothér. 2005, 9, 196–197. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, B.L.T.; Pijls-Johannesma, M.; Joore, M.A.; van den Ende, P.; Langendijk, J.A.; Lambin, P.; Kessels, A.G.H.; Grutters, J.P.C. Systematic review and meta-analysis of radiotherapy in various head and neck cancers: Comparing photons, carbon-ions and protons. Cancer Treat. Rev. 2011, 37, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, M.F.; Bielenberg, D.R.; Lenormand, G.; Marinkovic, M.; Waghorne, C.G.; Zetter, B.R.; Fredberg, J.J. Cytoskeletal stiffness, friction, and fluidity of cancer cell lines with different metastatic potential. Clin. Exp. Metastasis 2013, 30, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Doornaert, B.; Leblond, V.; Planus, E.; Galiacy, S.; Laurent, V.M.; Gras, G.; Isabey, D.; Lafuma, C. Time course of actin cytoskeleton stiffness and matrix adhesion molecules in human bronchial epithelial cell cultures. Exp. Cell Res. 2003, 287, 199–208. [Google Scholar] [CrossRef]
- Suresh, S. Nanomedicine: Elastic clues in cancer detection. Nat. Nanotechnol. 2007, 2, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Jin, Y.-S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Jin, Y.-S.; Tondre, J.; Wong, R.; Rao, J.; Gimzewski, J.K. AFM-based analysis of human metastatic cancer cells. Nanotechnology 2008, 19, 384003. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Lee, G.Y.H.; Ong, C.N.; Lim, C.T. AFM indentation study of breast cancer cells. Biochem. Biophys. Res. Commun. 2008, 374, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Guck, J.; Schinkinger, S.; Lincoln, B.; Wottawah, F.; Ebert, S.; Romeyke, M.; Lenz, D.; Erickson, H.M.; Ananthakrishnan, R.; Mitchell, D.; et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J. 2005, 88, 3689–3698. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Weaver, V.M. Mechanics, malignancy, and metastasis: The force journey of a tumor cell. Cancer Metastasis Rev. 2009, 28, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, A.; Höckel, M.; Kiessling, T.; Nnetu, K.D.; Wetzel, F.; Zink, M.; Käs, J.A. Are biomechanical changes necessary for tumour progression? Nat. Phys. 2010, 6, 730–732. [Google Scholar] [CrossRef]
- Bassi, C.; Mello, S.S.; Cardoso, R.S.; Godoy, P.D.; Fachin, A.L.; Junta, C.M.; Sandrin-Garcia, P.; Carlotti, C.G.; Falcao, R.P.; Donadi, E.A.; et al. Transcriptional changes in U343 MG-a glioblastoma cell line exposed to ionizing radiation. Hum. Exp. Toxicol. 2008, 27, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.R.D.V.; Mello, S.S.; Magalhães, D.A.R.; Donaires, F.S.; Nicolucci, P.; Donadi, E.A.; Passos, G.A.; Sakamoto-Hojo, E.T. Ionizing radiation-induced gene expression changes in TP53 proficient and deficient glioblastoma cell lines. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 756, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wang, H.; Ma, H.; Wang, H.; Chen, B.; Deng, Y. Starvation after Cobalt-60 γ-Ray Radiation Enhances Metastasis in U251 Glioma Cells by Regulating the Transcription Factor SP1. Int. J. Mol. Sci. 2016, 17, 386. [Google Scholar] [CrossRef] [PubMed]
- Gabryś, D.; Greco, O.; Patel, G.; Prise, K.M.; Tozer, G.M.; Kanthou, C. Radiation Effects on the Cytoskeleton of Endothelial Cells and Endothelial Monolayer Permeability. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Cordes, N.; Hansmeier, B.; Beinke, C.; Meineke, V.; van Beuningen, D. Irradiation differentially affects substratum-dependent survival, adhesion, and invasion of glioblastoma cell lines. Br. J. Cancer 2003, 89, 2122–2132. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Jung, J.W.; Jeong, J.S.; Kim, Y.J.; Oh, E.S.; Kim, T.H.; Kim, J.Y.; Cho, K.H.; Han, I.O. Dominant-negative Rac increases both inherent and ionizing radiation-induced cell migration in C6 rat glioma cells. Int. J. Cancer 2006, 118, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, T.; Grabiec, U.; Ghadban, C.; Feese, K.; Dehghani, F. The Influence of Biomechanical Properties and Cannabinoids on Tumor Invasion. Cell Adh. Migr. 2017, 11, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Van Meir, E.; Kikuchi, T.; Tada, M.; Li, H.; Diserens, A.; Wojcik, B.; Huang, S.; Friedmann, T.; de Tribolet, N.; Cavenee, W. Analysis of the p53 Gene and Its Expression in Human Glioblastoma. Cancer Res. 1994, 54, 649–652. [Google Scholar] [PubMed]
- James, C.D. Editorial: P53 in malignant glioma: 20 years later and still much to learn. Neuro Oncol. 2010, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Moritake, T.; Tsuchida, Y.; Tokuuye, K.; Marsumora, A.; Ando, K. Cell Cycle Checkpoint and Apoptosis Induction in Glioblastoma Cells and Fibroblasts Irradiated with Carbon Beam. J. Radiat. Res. 2007, 48, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Badie, B.; Goh, C.S.; Klaver, J.; Herweijer, H.; Boothman, D.A. Combined radiation and p53 gene therapy of malignant glioma cells. Cancer Gene Ther. 1999, 6, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Tsuchida, Y.; Keng, P.C. Effects of p53 mutations on cellular sensitivity to ionizing radiation. Am. J. Clin. Oncol. 2001, 24, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Nieder, C.; Petersen, S.; Petersen, C.; Thames, H.D. The challenge of p53 as prognostic and predictive factor in gliomas. Cancer Treat. Rev. 2000, 26, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Cuddihy, A.R.; Bristow, R.G. The p53 protein family and radiation sensitivity: Yes or no? Cancer Metastasis Rev. 2004, 23, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Jo, G.H.; Bögler, O.; Chwae, Y.J.; Yoo, H.; Lee, S.H.; Park, J.B.; Kim, Y.J.; Kim, J.H.; Gwak, H.S. Radiation-induced autophagy contributes to cell death and induces apoptosis partly in malignant glioma cells. Cancer Res. Treat. 2015, 47, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Bohl, J.; Elsasser, T.; Weber, K.-J.; Schulz-Ertner, D.; Debus, J.; Weyrather, W.K. Radiobiological evaluation and correlation with the local effect model (LEM) of carbon ion radiation therapy and temozolomide in glioblastoma cell lines. Int. J. Radiat. Biol. 2009, 85, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.; Guettler, A.; Wichmann, H.; Rot, S.; Kappler, M.; Bache, M.; Vordermark, D. IDH1R132H mutation causes a less aggressive phenotype and radiosensitizes human malignant glioma cells independent of the oxygenation status. Radiother. Oncol. 2015, 116, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Paolini, A.; Pasi, F.; Facoetti, A.; Mazzini, G.; Corbella, F.; Di Liberto, R.; Nano, R. Cell death forms and HSP70 expression in U87 cells after ionizing radiation and/or chemotherapy. Anticancer Res. 2011, 31, 3727–3731. [Google Scholar] [PubMed]
- Hong, X.; Chedid, K.; Kalkanis, S.N. Glioblastoma cell line-derived spheres in serum-containing medium versus serum-free medium: A comparison of cancer stem cell properties. Int. J. Oncol. 2012, 41, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guo, Y.; Li, Y.; Li, W.; Zheng, X.; Xia, H.; Mao, Q. Detection of CD133 expression in U87 glioblastoma cells using a novel anti-CD133 monoclonal antibody. Oncol. Lett. 2015, 9, 2603–2608. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.G.; Malhotra, R.; Delaney, M.; Latham, D.; Nestler, U.; Zhang, M.; Mukherjee, N.; Song, Q.; Robe, P.; Chakravarti, A. Radiation enhances the invasive potential of primary glioblastoma cells via activation of the Rho signaling pathway. J. Neurooncol. 2006, 76, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Alapati, K.; Gopinath, S.; Malla, R.R.; Dasari, V.R.; Rao, J.S. uPAR and cathepsin B knockdown inhibits radiation-induced PKC integrated integrin signaling to the cytoskeleton of glioma-initiating cells. Int. J. Oncol. 2012, 41, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. Ras-related GTPases and the cytoskeleton. Mol. Biol. Cell 1992, 3, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Nobes, C.D.; Hall, A. Rho, rac and cdc42 GTPases: Regulators of actin structures, cell adhesion and motility Rac stimulates actin polymerization to form lamellipodia Addition of other growth factors to serum-starved Rho induces the assembly of actin stress fibres and focal. Biochem. Soc. Trans. 1995, 23, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Nobes, C.D.; Hall, A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995, 81, 53–62. [Google Scholar] [CrossRef]
- Ridley, A.J.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70, 389–399. [Google Scholar] [CrossRef]
- Ramis, G.; Thomàs-Moyà, E.; de Mattos, S.; Rodríguez, J.; Villalonga, P. EGFR inhibition in glioma cells modulates rho signaling to inhibit cell motility and invasion and cooperates with temozolomide to reduce cell growth. PLoS ONE 2012, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Fan, Z.; Ding, M.; Zhang, H.; Mu, L.; Ding, Y.; Zhang, Y.; Jia, B.; Chen, L.; Chang, Z.; et al. An EGFR /PI3K/AKT axis promotes accumulation of the Rac1-GEF Tiam1 that is critical in EGFR-driven tumorigenesis. Oncogene 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Coniglio, S.J.; Chan, A.; Symons, M.H.; Steinberg, B.M. Upregulation of Rac1 by Epidermal Growth Factor Mediates COX-2 Expression in Recurrent Respiratory Papillomas. Mol. Med. 2007, 13, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Stahler, C.; Roth, J.; Cordes, N.; Taucher-Scholz, G.; Mueller-Klieser, W. Impact of carbon ion irradiation on epidermal growth factor receptor signaling and glioma cell migration in comparison to conventional photon irradiation. Int. J. Radiat. Biol. 2013, 89, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Koch, D.; Cardenas, R.; Käs, J.; Shih, C.K. Cell Motility and Local Viscoelasticity of Fibroblasts. Biophys. J. 2005, 89, 4330–4342. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Spatz, J.P.; Bershadsky, A. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Panzetta, V.; De Menna, M.; Musella, I.; Pugliese, M.; Quarto, M.; Netti, P.A.; Fusco, S. X-rays effects on cytoskeleton mechanics of healthy and tumor cells. Cytoskeleton 2017, 74, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, L.; Li, Z.; Liu, Y.; Zhang, H.; Wang, J. Carbon Ion-Irradiated Hepatoma Cells Exhibit Coupling Interplay between Apoptotic Signaling and Morphological and Mechanical Remodeling. Sci. Rep. 2016, 6, 35131. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, V.; Mythreye, K.; Tim O’Brien, E.; Berchuck, A.; Blobe, G.C.; Superfine, R. Mechanical Stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011, 71, 5075–5080. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell Stiffness Is a Biomarker of the Metastatic Potential of Ovarian Cancer Cells. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kuramochi, H.; Takahashi, A.; Imai, K.; Katsuta, N.; Nakayama, T.; Fujiki, H.; Suganuma, M. Higher cell stiffness indicating lower metastatic potential in B16 melanoma cell variants and in (2)-epigallocatechin gallate-treated cells. J. Cancer Res. Clin. Oncol. 2012, 138, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Darling, E.M.; Zauscher, S.; Block, J.A.; Guilak, F. A Thin-Layer Model for Viscoelastic, Stress-Relaxation Testing of Cells Using Atomic Force Microscopy: Do Cell Properties Reflect Metastatic Potential? Biophys. J. 2007, 92, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Thoumine, O.; Ott, A. Comparison of the mechanical properties of normal and transformed fibroblasts. Biorheology 1997, 34, 309–326. [Google Scholar] [CrossRef]
- Faria, E.C.; Ma, N.; Gazi, E.; Gardner, P.; Brown, M.; Clarke, N.W.; Snook, R.D. Measurement of elastic properties of prostate cancer cells using AFM. Analyst 2008, 133, 1498. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Mater. 2007, 55, 3989–4014. [Google Scholar] [CrossRef]
- Suresh, S.; Spatz, J.; Mills, J.P.; Micoulet, A.; Dao, M.; Lim, C.T.; Beil, M.; Seufferlein, T. Reprint of: Connections between single-cell biomechanics and human disease states: Gastrointestinal cancer and malaria. Acta Biomater. 2015, 23, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Ochalek, T.; Nordt, F.J.; Tullberg, K.; Variants, M.C.; Burger, M.M. Correlation between Cell Deformability and Metastatic Potential in B16-F1 Melanoma Cell Variants. Cancer Res. 1988, 48, 5124–5128. [Google Scholar] [PubMed]
- Iyer, S.; Gaikwad, R.M.; Subba-Rao, V.; Woodworth, C.D.; Sokolov, I. AFM Detects Differences in the Surface Brush of Normal and Cancerous Cervical Cells. Nat. Nanotechnol. 2009, 4, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Lekka, M.; Pogoda, K.; Gostek, J.; Klymenko, O.; Prauzner-Bechcicki, S.; Wiltowska-Zuber, J.; Jaczewska, J.; Lekki, J.; Stachura, Z. Cancer cell recognition—Mechanical phenotype. Micron 2012, 43, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Lekka, M.; Gil, D.; Pogoda, K.; Dulińska-Litewka, J.; Jach, R.; Gostek, J.; Klymenko, O.; Prauzner-Bechcicki, S.; Stachura, Z.; Wiltowska-Zuber, J.; et al. Cancer cell detection in tissue sections using AFM. Arch. Biochem. Biophys. 2012, 518, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Remmerbach, T.W.; Wottawah, F.; Dietrich, J.; Lincoln, B.; Wittekind, C.; Guck, J. Oral cancer diagnosis by mechanical phenotyping. Cancer Res. 2009, 69, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Wachsberger, P.R.; Burd, R.; Marero, N.; Daskalakis, C.; Ryan, A.; McCue, P.; Dicker, A.P. Effect of the tumor vascular-damaging agent, ZD6126, on the radioresponse of U87 glioblastoma. Clin. Cancer Res. 2005, 11, 835–842. [Google Scholar] [PubMed]
- Canazza, A.; Calatozzolo, C.; Fumagalli, L.; Bergantin, A.; Ghielmetti, F.; Fariselli, L.; Croci, D.; Salmaggi, A.; Ciusani, E. Increased migration of a human glioma cell line after in vitro CyberKnife irradiation. Cancer Biol. Ther. 2011, 12, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Steinle, M.; Palme, D.; Misovic, M.; Rudner, J.; Dittmann, K.; Lukowski, R.; Ruth, P.; Huber, S.M. Ionizing radiation induces migration of glioblastoma cells by activating BK K + channels. Radiother. Oncol. 2011, 101, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Kumar, S.; Iskander, A.S.M.; Varma, N.R.S.; Janic, B.; deCarvalho, A.; Mikkelsen, T.; Frank, J.A.; Ali, M.M.; Knight, R.A.; et al. Subcurative radiation significantly increases cell proliferation, invasion, and migration of primary glioblastoma multiforme in vivo. Chin. J. Cancer 2014, 33, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhou, L.; Han, N.; Zhang, M.; Lyu, X. Wnt/β-catenin pathway involvement in ionizing radiation-induced invasion of U87 glioblastoma cells. Strahlenther. Onkol. 2015, 191, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.R.; Kirkpatrick, J.P.; Fiveash, J.B.; Shih, H.A.; Koay, E.J.; Lutz, S.; Petit, J.; Chao, S.T.; Brown, P.D.; Vogelbaum, M.; et al. Radiation therapy for glioblastoma: Executive summary of an American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. Pract. Radiat. Oncol. 2016, 6, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Falzone, T.T.; Lenz, M.; Kovar, D.R.; Gardel, M.L. Assembly Kinetics Determine the Architecture of α-actinin Crosslinked F-actin Networks. Nat. Commun. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.; Hoadley, K.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.; Miller, C.; Ding, L.; Golub, T.; Mesirov, J.; et al. An integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR and NF1. Cancer Cell 2010, 19, 38–46. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandris, Q.G.; Biffoni, M.; Martini, M.; Runci, D.; Buccarelli, M.; Cenci, T.; Signore, M.; Stancato, L.; Olivi, A.; De Maria, R.; et al. The clinical value of patient-derived glioblastoma tumorspheres in predicting treatment response. Neuro Oncol. 2017, 19, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Hezel, M.; Koch, M.; Ghadban, C.; Korf, H.W.; Dehghani, F. Analyses of neuronal damage in excitotoxically lesioned organotypic hippocampal slice cultures. Ann. Anat. 2010, 192, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of contact deformations on the adhesion of particles. Prog. Surf. Sci. 1975, 45, 131–143. [Google Scholar] [CrossRef]
- Grabiec, U.; Koch, M.; Kallendrusch, S.; Kraft, R.; Hill, K.; Merkwitz, C.; Ghadban, C.; Lutz, B.; Straiker, A.; Dehghani, F. The endocannabinoid N-arachidonoyldopamine (NADA) exerts neuroprotective effects after excitotoxic neuronal damage via cannabinoid receptor 1 (CB 1). Neuropharmacology 2012, 62, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, C.; Fuchs, S.; Monoranu, C.M.; Herrmann, P.; Smith, J.; Hohmann, T.; Grabiec, U.; Kessler, A.F.; Dehghani, F.; Lo, M.; et al. Impact of MACC1 on human malignant glioma progression and patients’ unfavorable prognosis. J. Neurooncol. 2013, 15, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Hezel, M.; Ebrahimi, F.; Koch, M.; Dehghani, F. Propidium iodide staining: A new application in fluorescence microscopy for analysis of cytoarchitecture in adult and developing rodent brain. Micron 2012, 43, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Grabiec, U.; Hohmann, T.; Hammer, N.; Dehghani, F. Organotypic Hippocampal Slice Cultures As a Model to Study Neuroprotection and Invasiveness of Tumor Cells. J. Vis. Exp. 2017, 126, e55359. [Google Scholar] [CrossRef] [PubMed]
- Weichsel, J.; Herold, N.; Lehmann, M.J.; Kräusslich, H.G.; Schwarz, U.S. A quantitative measure for alterations in the actin cytoskeleton investigated with automated high-throughput microscopy. Cytom. Part A 2010, 77, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Kießling, T.R.; Herrera, M.; Nnetu, K.D.; Balzer, E.M.; Girvan, M.; Fritsch, A.W.; Martin, S.S.; Käs, J.A.; Losert, W. Analysis of multiple physical parameters for mechanical phenotyping of living cells. Eur. Biophys. J. 2013, 42, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Newman, M. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577–8582. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohmann, T.; Grabiec, U.; Vogel, C.; Ghadban, C.; Ensminger, S.; Bache, M.; Vordermark, D.; Dehghani, F. The Impact of Non-Lethal Single-Dose Radiation on Tumor Invasion and Cytoskeletal Properties. Int. J. Mol. Sci. 2017, 18, 2001. https://doi.org/10.3390/ijms18092001
Hohmann T, Grabiec U, Vogel C, Ghadban C, Ensminger S, Bache M, Vordermark D, Dehghani F. The Impact of Non-Lethal Single-Dose Radiation on Tumor Invasion and Cytoskeletal Properties. International Journal of Molecular Sciences. 2017; 18(9):2001. https://doi.org/10.3390/ijms18092001
Chicago/Turabian StyleHohmann, Tim, Urszula Grabiec, Carolin Vogel, Chalid Ghadban, Stephan Ensminger, Matthias Bache, Dirk Vordermark, and Faramarz Dehghani. 2017. "The Impact of Non-Lethal Single-Dose Radiation on Tumor Invasion and Cytoskeletal Properties" International Journal of Molecular Sciences 18, no. 9: 2001. https://doi.org/10.3390/ijms18092001
APA StyleHohmann, T., Grabiec, U., Vogel, C., Ghadban, C., Ensminger, S., Bache, M., Vordermark, D., & Dehghani, F. (2017). The Impact of Non-Lethal Single-Dose Radiation on Tumor Invasion and Cytoskeletal Properties. International Journal of Molecular Sciences, 18(9), 2001. https://doi.org/10.3390/ijms18092001