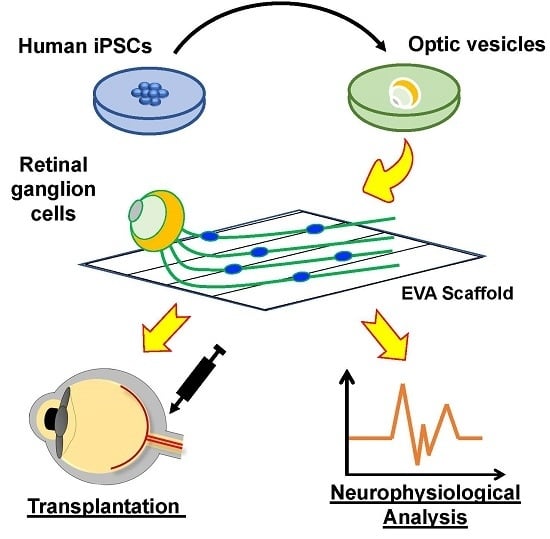
Elongation of Axon Extension for Human iPSC-Derived Retinal Ganglion Cells by a Nano-Imprinted Scaffold
Abstract
:1. Introduction
2. Results
2.1. Generation of OVs from Human iPSCs
2.2. Functional Analysis of Human iPSC-Derived Rgcs
2.3. The Poly(ethylene-co-vinyl acetate) Material into a Straight Groove via Nano-Imprinting Lithography
2.4. Groove Scaffold Guides RGC’s Neurite Outgrowth
2.5. Groove Scaffold Promotes RGC Growth in Patient-Derived Cells with RGC Degeneration Disease
2.6. Summary
3. Discussion
4. Materials and Methods
4.1. Induction of hiPSC-Derived RGCs
4.2. Immunofluorescence
4.3. Electrophysiological Analysis
4.4. Nanoimprinting of Topographical Scaffolds for Cell Culturing
4.5. Characterization of Topographical Cell Culture Scaffolds
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LHON | Leber’s hereditary optic neuropathy |
| RGC | Retinal ganglion cells |
| iPSC | Induced pluripotent stem cell |
| OVs | Optic vesicles |
| EVA | Poly(ethylene-co-vinyl acetate) |
| T-NIL | Thermal nano-imprinting lithography |
| SEM | Scanning electron microscopy |
| AFM | Atomic force microscopy |
References
- So, K.F.; Yip, H.K. Regenerative capacity of retinal ganglion cells in mammals. Vis. Res. 1998, 38, 1525–1535. [Google Scholar] [CrossRef]
- Richardson, P.M.; Issa, V.M.; Shemie, S. Regeneration and retrograde degeneration of axons in the rat optic nerve. J. Neurocytol. 1982, 11, 949–966. [Google Scholar] [CrossRef] [PubMed]
- Grafstein, B.; Ingoglia, N.A. Intracranial transection of the optic nerve in adult mice: Preliminary observations. Exp. Neurol. 1982, 76, 318–330. [Google Scholar] [CrossRef]
- Wong, I.Y.; Poon, M.W.; Pang, R.T.; Lian, Q.; Wong, D. Promises of stem cell therapy for retinal degenerative diseases. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1439–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, L.S.; Phillips, M.J.; Pinilla, I.; Hei, D.; Gamm, D.M. Induced pluripotent stem cells as custom therapeutics for retinal repair: Progress and rationale. Exp. Eye Res. 2014, 123, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Kumar Saha, S.; Yang, G.M.; Choi, H.Y.; Cho, S.G. Recent Advances in Disease Modeling and Drug Discovery for Diabetes Mellitus Using Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2016, 17, 256. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Induced pluripotent stem cells: Past, present, and future. Cell Stem Cell 2012, 10, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Durnaoglu, S.; Genc, S.; Genc, K. Patient-specific pluripotent stem cells in neurological diseases. Stem Cells Int. 2011, 2011, 212487. [Google Scholar] [CrossRef] [PubMed]
- Dianat, N.; Steichen, C.; Vallier, L.; Weber, A.; Dubart-Kupperschmitt, A. Human pluripotent stem cells for modelling human liver diseases and cell therapy. Curr. Gene Ther. 2013, 13, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.S.; Howden, S.E.; Wallace, K.A.; Verhoeven, A.D.; Wright, L.S.; Capowski, E.E.; Pinilla, I.; Martin, J.M.; Tian, S.; Stewart, R.; et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells 2011, 29, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Doorenbos, M.; Chen, D.F. Induced Pluripotent Stem Cells: Development in the Ophthalmologic Field. Stem Cells Int. 2016, 2016, 2361763. [Google Scholar] [CrossRef] [PubMed]
- Whited, B.M.; Rylander, M.N. The influence of electrospun scaffold topography on endothelial cell morphology, alignment, and adhesion in response to fluid flow. Biotechnol. Bioeng. 2014, 111, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Adachi, T. Topography Design Concept of a Tissue Engineering Scaffold for Controlling Cell Function and Fate through Actin Cytoskeletal Modulation. Tissue Eng. Part B Rev. 2014, 20, 609–627. [Google Scholar] [CrossRef] [PubMed]
- Seol, Y.-J.; Kang, H.-W.; Lee, S.J.; Atala, A.; Yoo, J.J. Bioprinting technology and its applications. Eur. J. Cardio-Thorac. Surg. 2014, 46, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.; van Langeveld, M.C.; Donoso, L.A. Innovations in 3D printing: A 3D overview from optics to organs. Br. J. Ophthalmol. 2014, 98, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Kador, K.E.; Grogan, S.P.; Dorthe, E.W.; Venugopalan, P.; Malek, M.F.; Goldberg, J.L.; D’Lima, D.D. Control of Retinal Ganglion Cell Positioning and Neurite Growth: Combining 3D Printing with Radial Electrospun Scaffolds. Tissue Eng. Part A 2016, 22, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Chen, M.; Le, D.Q.; Baatrup, A.; Nygaard, J.V.; Hein, S.; Bjerre, L.; Kassem, M.; Zou, X.; Bunger, C. Self-assembled composite matrix in a hierarchical 3-D scaffold for bone tissue engineering. Acta Biomater. 2011, 7, 2244–2255. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.J.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Polymer concepts in tissue engineering. J. Biomed. Mater. Res. 1998, 43, 422–427. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Johansson, F.; Carlberg, P.; Danielsen, N.; Montelius, L.; Kanje, M. Axonal outgrowth on nano-imprinted patterns. Biomaterials 2006, 27, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, M.J.; Chen, R.R.; Tan, J.; Saltzman, W.M. The influence of microchannels on neurite growth and architecture. Biomaterials 2005, 26, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Mattotti, M.; Alvarez, Z.; Ortega, J.A.; Planell, J.A.; Engel, E.; Alcantara, S. Inducing functional radial glia-like progenitors from cortical astrocyte cultures using micropatterned PMMA. Biomaterials 2012, 33, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, R.; Doevendans, P.A.; Metz, C.H.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J.P. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 2012, 33, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Tang-Schomer, M.D.; White, J.D.; Tien, L.W.; Schmitt, L.I.; Valentin, T.M.; Graziano, D.J.; Hopkins, A.M.; Omenetto, F.G.; Haydon, P.G.; Kaplan, D.L. Bioengineered functional brain-like cortical tissue. Proc. Natl. Acad. Sci. USA 2014, 111, 13811–13816. [Google Scholar] [CrossRef] [PubMed]
- Worthington, K.S.; Wiley, L.A.; Kaalberg, E.E.; Collins, M.M.; Mullins, R.F.; Stone, E.M.; Tucker, B.A. Two-photon polymerization for production of human iPSC-derived retinal cell grafts. Acta Biomater. 2017, 55, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Lorber, B.; Hsiao, W.K.; Hutchings, I.M.; Martin, K.R. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication 2014, 6, 015001. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Hu, Z.; Zhang, X.; Li, X.; Gao, Z.; Yuan, D.; Liu, Q. Application of 3-dimensional printing technology to construct an eye model for fundus viewing study. PLoS ONE 2014, 9, e109373. [Google Scholar] [CrossRef] [PubMed]
- Ohlemacher, S.K.; Sridhar, A.; Xiao, Y.; Hochstetler, A.E.; Sarfarazi, M.; Cummins, T.R.; Meyer, J.S. Stepwise Differentiation of Retinal Ganglion Cells from Human Pluripotent Stem Cells Enables Analysis of Glaucomatous Neurodegeneration. Stem Cells 2016, 34, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yokoi, T.; Tamalu, F.; Watanabe, S.; Nishina, S.; Azuma, N. Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Sci. Rep. 2015, 5, 8344. [Google Scholar] [CrossRef] [PubMed]
- Roumen, F.J.; Dieben, T.O. Clinical acceptability of an ethylene-vinyl-acetate nonmedicated vaginal ring. Contraception 1999, 59, 59–62. [Google Scholar] [CrossRef]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Kenawy el, R.; Layman, J.M.; Watkins, J.R.; Bowlin, G.L.; Matthews, J.A.; Simpson, D.G.; Wnek, G.E. Electrospinning of poly(ethylene-co-vinyl alcohol) fibers. Biomaterials 2003, 24, 907–913. [Google Scholar] [CrossRef]
- Stitzel, J.; Bowlin, G.; Wnek, G.; Simpson, D.G. Electrospraying and electrospinning of polymers for biomedical applications. Poly(lactic-co-glycolic acid) and poly(ethylene-co-vinylacetate). In Proceedings of the 32nd SAMPE Meeting, Boston, MA, USA, 5–9 November 2000; Volume 32. [Google Scholar]
- Genina, N.; Hollander, J.; Jukarainen, H.; Makila, E.; Salonen, J.; Sandler, N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur. J. Pharm. Sci. 2016, 90, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.C.; Haider, A.; Choi, Y.-R.; Kang, I.-K. Nanofibrous scaffolds in biomedical applications. Biomater. Res. 2014, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Kalachandra, S.; Takamata, T.; Lin, D.M.; Snyder, E.A.; Webster-Cyriaque, J. Stability and release of antiviral drugs from ethylene vinyl acetate (EVA) copolymer. J. Mater. Sci. Mater. Med. 2006, 17, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Kenawy el, R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Shi, X.; Fujie, T.; Saito, A.; Takeoka, S.; Hou, Y.; Shu, Y.; Chen, M.; Wu, H.; Khademhosseini, A. Periosteum-mimetic structures made from freestanding microgrooved nanosheets. Adv. Mater. 2014, 26, 3290–3296. [Google Scholar] [CrossRef] [PubMed]
- Sluch, V.M.; Davis, C.H.; Ranganathan, V.; Kerr, J.M.; Krick, K.; Martin, R.; Berlinicke, C.A.; Marsh-Armstrong, N.; Diamond, J.S.; Mao, H.Q.; et al. Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Sci. Rep. 2015, 5, 16595. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, T.; Yin, Y.; Omura, K.; Gilbert, H.-Y.; Kim, D.; Cen, L.-P.; Moko, L.; Kugler, S.; Benowitz, L.I. Long-distance axon regeneration in the mature optic nerve: Contributions of Oncomodulin, cAMP, and pten gene deletion. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 15654–15663. [Google Scholar] [CrossRef] [PubMed]








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.-C.; Chuang, J.-H.; Buddhakosai, W.; Wu, W.-J.; Lee, C.-J.; Chen, W.-S.; Yang, Y.-P.; Li, M.-C.; Peng, C.-H.; Chen, S.-J. Elongation of Axon Extension for Human iPSC-Derived Retinal Ganglion Cells by a Nano-Imprinted Scaffold. Int. J. Mol. Sci. 2017, 18, 2013. https://doi.org/10.3390/ijms18092013
Yang T-C, Chuang J-H, Buddhakosai W, Wu W-J, Lee C-J, Chen W-S, Yang Y-P, Li M-C, Peng C-H, Chen S-J. Elongation of Axon Extension for Human iPSC-Derived Retinal Ganglion Cells by a Nano-Imprinted Scaffold. International Journal of Molecular Sciences. 2017; 18(9):2013. https://doi.org/10.3390/ijms18092013
Chicago/Turabian StyleYang, Tien-Chun, Jen-Hua Chuang, Waradee Buddhakosai, Wen-Ju Wu, Chen-Ju Lee, Wun-Syuan Chen, Yi-Ping Yang, Ming-Chia Li, Chi-Hsien Peng, and Shih-Jen Chen. 2017. "Elongation of Axon Extension for Human iPSC-Derived Retinal Ganglion Cells by a Nano-Imprinted Scaffold" International Journal of Molecular Sciences 18, no. 9: 2013. https://doi.org/10.3390/ijms18092013
APA StyleYang, T. -C., Chuang, J. -H., Buddhakosai, W., Wu, W. -J., Lee, C. -J., Chen, W. -S., Yang, Y. -P., Li, M. -C., Peng, C. -H., & Chen, S. -J. (2017). Elongation of Axon Extension for Human iPSC-Derived Retinal Ganglion Cells by a Nano-Imprinted Scaffold. International Journal of Molecular Sciences, 18(9), 2013. https://doi.org/10.3390/ijms18092013