Chemical Exposure-Induced Changes in the Expression of Neurotrophins and Their Receptors in the Main Olfactory System of Mice Lacking TRPM5-Expressing Microvillous Cells
Abstract
:1. Introduction
2. Results
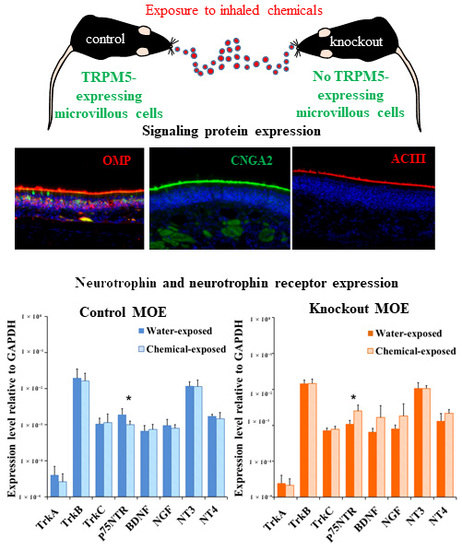
2.1. Immunolabeling of Olfactory Signaling Proteins in Vehicle- and Chemical-Exposed TRPM5-GFP and Skn-1a−/− Mice
2.2. Quantitative Analysis of the Expression of NT and NTR Gene Transcripts in the MOE and OB of Vehicle-Exposed Skn-1a−/− and TRPM5-GFP Mice
2.3. Changes in the Expression Levels of NTs and NTRs in the MOE of TRPM5-GFP and Skn-1a−/− Mice after a Two-Week Chemical Exposure
2.4. Regional Changes in the Expression Levels of NTs and NTRs after a Two-Week Chemical Exposure in the Anterior and Posterior MOE of TRPM5-GFP and Skn-1a−/− Mice
2.5. Changes in the Expression of NTs and NTRs in the OB of TRPM5-GFP and Skn-1a−/− Mice after Two-Week Chemical Exposure
2.6. RISH Analysis of the Spatial Distribution of NT and NTR Gene Transcripts in the MOE
2.7. Immunolabeling of p75NTR
3. Discussion
3.1. Expression of NTs and NTRs in the MOE and OB of the TRPM5-GFP and Skn-1a−/− Mice Exposed to Vehicle (Water)
3.2. Changes in the Expression Levels of NTs and NTRs in the MOE and OB of Chemical-Exposed TRPM5-GFP and Skn-1a−/− Mice
3.3. Discrepancy Between Different Approaches and Impact of Chemical Exposure Strength.
4. Materials and Methods
4.1. Animals
4.2. Chemical Exposure
4.3. Immunocytochemistry
4.4. Real-Time Quantitative PCR
4.5. RISH Experiments
4.6. Image Acquisition
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | Acetylcholine |
| ANOVA | Analysis of variance |
| BCIP | 5-bromo-4-chloro-3′-indolyl phosphate |
| BDNF | Brain-derived neurotrophic factor |
| ERK | Extracellular signal-regulated protein kinase |
| GFP | Green fluorescent protein |
| HBC | Horizontal basal cell |
| MC | Microvillous cell |
| MOE | Main olfactory epithelium |
| NBT | Nitro blue tetrazolium |
| NGF | Nerve growth factor |
| NT | Neurotrophin |
| NT-3 | Neurotrophin-3 |
| NT-4 | Neurotrophin-4 |
| NTR | NT receptor |
| OB | Olfactory bulb |
| OSN | Olfactory sensory neuron |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| PLCγ | Phospholipase Cγ |
| qPCR | Quantitative polymerase chain reaction |
| SC | Supporting cell |
| SD | Standard deviation |
| RISH | RNA in situ hybridization |
| RT-PCR | Reverse transcription polymerase chain reaction |
| Trk | Tropomyosin receptor kinase |
| TRPC6 | Transient receptor potential channel C6 |
| TRPM5-MC | Transient receptor potential channel M5-expressing microvillous cell |
References
- Ajmani, G.S.; Suh, H.H.; Pinto, J.M. Effects of Ambient Air Pollution Exposure on Olfaction: A Review. Environ. Health Perspect. 2016, 124, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, R.G.; Dorman, D.C.; Elder, A.; Veronesi, B. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology 2012, 33, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus infections in the nervous system. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. The olfactory vector hypothesis of neurodegenerative disease: Is it viable? Ann. Neurol. 2008, 63, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willner, P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005, 52, 90–110. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Hayoz, S.; Hutch, C.R.; Iqbal, T.R.; Pooley, A.E.; Hegg, C.C. An IP3R3- and NPY-expressing microvillous cell mediates tissue homeostasis and regeneration in the mouse olfactory epithelium. PLoS ONE 2013, 8, e58668. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Ezekwe, E.A., Jr.; Zhao, Z.; Liman, E.R.; Restrepo, D. TRPM5-expressing microvillous cells in the main olfactory epithelium. BMC Neurosci. 2008, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Finger, T.E. Is TrpM5 a reliable marker for chemosensory cells? Multiple types of microvillous cells in the main olfactory epithelium of mice. BMC Neurosci. 2008, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Szebenyi, S.A.; Krosnowski, K.; Sathyanesan, A.; Jackson, J.; Lin, W. Cholinergic microvillous cells in the mouse main olfactory epithelium and effect of acetylcholine on olfactory sensory neurons and supporting cells. J. Neurophysiol. 2011, 106, 1274–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Z.; Ogura, T.; Luo, W.; Lin, W. ATP and Odor Mixture Activate TRPM5-Expressing Microvillous Cells and Potentially Induce Acetylcholine Release to Enhance Supporting Cell Endocytosis in Mouse Main Olfactory Epithelium. Front. Cell. Neurosci. 2018, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Yamashita, J.; Ohmoto, M.; Aoudé, I.; Ogura, T.; Luo, W.; Bachmanov, A.A.; Lin, W.; Matsumoto, I.; Hirota, J. Skn-1a/Pou2f3 is required for the generation of Trpm5-expressing microvillous cells in the mouse main olfactory epithelium. BMC Neurosci. 2014, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Lemons, K.; Fu, Z.; Aoudé, I.; Ogura, T.; Sun, J.; Chang, J.; Mbonu, K.; Matsumoto, I.; Arakawa, H.; Lin, W. Lack of TRPM5-Expressing Microvillous Cells in Mouse Main Olfactory Epithelium Leads to Impaired Odor-Evoked Responses and Olfactory-Guided Behavior in a Challenging Chemical Environment. eNeuro 2017. [Google Scholar] [CrossRef] [PubMed]
- Farbman, A.I. Olfactory neurogenesis: Genetic or environmental controls? Trend. Neurosci. 1990, 13, 362–365. [Google Scholar] [CrossRef]
- Leung, C.T.; Coulombe, P.A.; Reed, R.R. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat. Neurosci. 2007, 10, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Weng, P.L.; Vinjamuri, M.; Ovitt, C.E. Ascl3 transcription factor marks a distinct progenitor lineage for non-neuronal support cells in the olfactory epithelium. Sci. Rep. 2016, 6, 38199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwob, J.E.; Jang, W.; Holbrook, E.H.; Lin, B.; Herrick, D.B.; Peterson, J.N.; Hewitt Coleman, J. Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license. J. Comp. Neurol. 2017, 525, 1034–1054. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Bailey, M.S.; Pixley, S.K.; Ennis, M.; Liu, W.; Shipley, M.T. Localization and regulation of low affinity nerve growth factor receptor expression in the rat olfactory system during development and regeneration. J. Comp. Neurol. 1994, 344, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Miwa, T.; Moriizumi, T.; Horikawa, I.; Uramoto, N.; Ishimaru, T.; Nishimura, T.; Furukawa, M. Role of nerve growth factor in the olfactory system. Microsc. Res. Tech. 2002, 58, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Mackay-Sima, A.; Chuahb, M.I. Neurotrophic factors in the primary olfactory pathway. Prog. Neurobiol. 2000, 62, 527–559. [Google Scholar] [CrossRef]
- Feron, F.; Bianco, J.; Ferguson, I.; Mackay-Sim, A. Neurotrophin expression in the adult olfactory epithelium. Brain Res. 2008, 1196, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Steuer, E.; Schaefer, M.L.; Belluscio, L. Using the olfactory system as an in vivo model to study traumatic brain injury and repair. J. Neurotrauma 2014, 31, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev. Neurosci. 1995, 18, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Barbacid, M. The Trk family of neurotrophin receptors. J. Neurobiol. 1994, 25, 1386–1403. [Google Scholar] [CrossRef] [PubMed]
- Maness, L.M.; Kastin, A.J.; Weber, J.T.; Banks, W.A.; Beckman, B.S.; Zadina, J.E. The neurotrophins and their receptors: Structure, function, and neuropathology. Neurosci. Biobehav. Rev. 1994, 18, 143–159. [Google Scholar] [CrossRef]
- Chao, M.V. The p75 neurotrophin receptor. J. Neurobiol. 1994, 25, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Szebenyi, S.A.; Ogura, T.; Sathyanesan, A.; AlMatrouk, A.K.; Chang, J.; Lin, W. Increases in intracellular calcium via activation of potentially multiple phospholipase C isozymes in mouse olfactory neurons. Front. Cell. Neurosci. 2014, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Ukhanov, K.; Corey, E.; Ache, B.W. Phosphoinositide-3-Kinase Is the Primary Mediator of Phosphoinositide-Dependent Inhibition in Mammalian Olfactory Receptor Neurons. Front. Cell. Neurosci. 2016, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Ukhanov, K.; Corey, E.A.; Brunert, D.; Klasen, K.; Ache, B.W. Inhibitory odorant signaling in Mammalian olfactory receptor neurons. J. Neurophysiol. 2010, 103, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Mammen, A.; Yoo, S.J.; Cho, B.; Kim, E.K.; Park, J.I.; Moon, C.; Ronnett, G.V. Phosphoinositide and Erk signaling pathways mediate activity-driven rodent olfactory sensory neuronal survival and stress mitigation. J. Neurochem. 2015, 134, 486–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, L.A.; Roskams, A.J. Neurotrophins and their receptors in the primary olfactory neuraxis. Microsc. Res. Tech. 2002, 58, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Takami, S.; Hasegawa, R.; Nishiyama, F. The roles of brain-derived neurotrophic factor in the development of nasal chemoreceptor neurons. Chem. Senses 2005, 30, i121–i122. [Google Scholar] [CrossRef] [PubMed]
- Buckland, M.E.; Cunningham, A.M. Alterations in expression of the neurotrophic factors glial cell line-derived neurotrophic factor, ciliary neurotrophic factor and brain-derived neurotrophic factor, in the target-deprived olfactory neuroepithelium. Neuroscience 1999, 90, 333–347. [Google Scholar] [CrossRef]
- Hagg, T. From neurotransmitters to neurotrophic factors to neurogenesis. Neuroscientist 2009, 15, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ueha, R.; Ueha, S.; Sakamoto, T.; Kanaya, K.; Suzukawa, K.; Nishijima, H.; Kikuta, S.; Kondo, K.; Matsushima, K.; Yamasoba, T. Cigarette Smoke Delays Regeneration of the Olfactory Epithelium in Mice. Neurotox. Res. 2016, 30, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Alexeeff, G.V.; Broadwin, R.; Salmon, A.G. Evaluation and application of the RD50 for determining acceptable exposure levels of airborne sensory irritants for the general public. Environ. Health Perspect 2007, 115, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Boase, S.; Foreman, A.; Cleland, E.; Tan, L.; Melton-Kreft, R.; Pant, H.; Hu, F.Z.; Ehrlich, G.D.; Wormald, P.J. The microbiome of chronic rhinosinusitis: Culture, molecular diagnostics and biofilm detection. BMC Infect. Dis. 2013, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Sigsgaard, T.; Thorne, P.S.; Schlunssen, V.; Bonlokke, J.; Riddervold, I.S.; Hoppe, K.A.; Andersen, N.T.; Mackenzie, N.M. The change in nasal inflammatory markers after intranasal challenges with particulate chitin and lipopolysaccharide: A randomized, double-blind, placebo-controlled, crossover study with a positive control. Int. Forum Allergy Rhinol. 2015, 5, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Sathyanesan, A.; Feijoo, A.A.; Mehta, S.T.; Nimarko, A.F.; Lin, W. Expression profile of G-protein βγ subunit gene transcripts in the mouse olfactory sensory epithelia. Front. Cell. Neurosci. 2013, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ponissery-Saidu, S.; Yee, K.K.; Wang, H.; Chen, M.L.; Iguchi, N.; Zhang, G.; Jiang, P.; Reisert, J.; Huang, L. Heterotrimeric G protein subunit Gγ13 is critical to olfaction. J. Neurosci. 2013, 33, 7975–7984. [Google Scholar] [CrossRef] [PubMed]
- Miwa, T.; Uramoto, N.; Ishimaru, T.; Furukawa, M.; Shiba, K.; Morjizumi, T. Retrograde transport of nerve growth factor from olfactory bulb to olfactory epithelium. Neuroreport 1998, 9, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, M.; Guthrie, K.M. Anterograde trafficking of neurotrophin-3 in the adult olfactory system in vivo. Exp. Neurol. 2013, 241, 125–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Challis, R.C.; Tian, H.; Wang, J.; He, J.; Jiang, J.; Chen, X.; Yin, W.; Connelly, T.; Ma, L.; Yu, C.R.; et al. An Olfactory Cilia Pattern in the Mammalian Nose Ensures High Sensitivity to Odors. Curr. Biol. 2015, 25, 2503–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, G.K.; Willson, C.J.; Olivera, D.S.; Malarkey, D.E.; Morgan, D.L. Comparative inhalation toxicity of ethyltoluene isomers in rats and mice. Inhal. Toxicol. 2017, 29, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Kenchappa, R.S.; Zampieri, N.; Chao, M.V.; Barker, P.A.; Teng, H.K.; Hempstead, B.L.; Carter, B.D. Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron 2006, 50, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.H.; Li, S.H.; Gao, Z.; Zou, S.F.; Li, H.Y.; Tao, Z.Y.; Song, J.; Yang, J.X. Neurotrophin-3 promotes proliferation and cholinergic neuronal differentiation of bone marrow- derived neural stem cells via notch signaling pathway. Life Sci. 2016, 166, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Calamandrei, G.; Alleva, E. Neuronal growth factors, neurotrophins and memory deficiency. Behav Brain Res. 1995, 66, 129–132. [Google Scholar] [CrossRef]
- Meeker, R.; Williams, K. Dynamic nature of the p75 neurotrophin receptor in response to injury and disease. J. Neuroimmune Pharmacol. 2014, 9, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, L.; Sun, D.; Li, J.; Yao, X.; Zhou, H.; Wang, Y. Roles of p75NTR in Maintaining Brain Hemostasis and the Implications for p75NTR-targeted Therapies. Curr. Alzheimer Res. 2017, 14, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Barker, P.A. p75NTR is positively promiscuous: Novel partners and new insights. Neuron 2004, 42, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.F.; Li, E.; Huber, L.J.; Landis, S.C.; Sharpe, A.H.; Chao, M.V.; Jaenisch, R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell 1992, 69, 737–749. [Google Scholar] [CrossRef]
- Nykjaer, A.; Lee, R.; Teng, K.K.; Jansen, P.; Madsen, P.; Nielsen, M.S.; Jacobsen, C.; Kliemannel, M.; Schwarz, E.; Willnow, T.E.; et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004, 427, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Dunston, D.; Ashby, S.; Krosnowski, K.; Ogura, T.; Lin, W. An effective manual deboning method to prepare intact mouse nasal tissue with preserved anatomical organization. J. Vis. Exp. 2013, 78, 50538. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, A.C.; Salcedo, E.; Jones, K.R.; Restrepo, D. BDNF promoter-mediated β-galactosidase expression in the olfactory epithelium and bulb. Chem. Senses 2008, 33, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Mishra, A. Olfaction and its alteration by nasal obstruction, rhinitis, and rhinosinusitis. Laryngoscope 2001, 111, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.J. Gulf War illness: An overview of events, most prevalent health outcomes, exposures, and clues as to pathogenesis. Rev. Environ. Health 2015, 30, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, I.; Ohmoto, M.; Narukawa, M.; Yoshihara, Y.; Abe, K. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat. Neurosci. 2011, 14, 685–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clapp, T.R.; Medler, K.F.; Damak, S.; Margolskee, R.F.; Kinnamon, S.C. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Margolskee, R.; Donnert, G.; Hell, S.W.; Restrepo, D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc. Natl. Acad. Sci. USA 2007, 104, 2471–2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Thriel, C.; Kiesswetter, E.; Schaper, M.; Juran, S.A.; Blaszkewicz, M.; Kleinbeck, S. Odor annoyance of environmental chemicals: Sensory and cognitive influences. J. Toxicol. Environ. Health A 2008, 71, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.J.; Fernandez, J.; Sohn, J.J.; Amemiya, C.T. Chitin is endogenously produced in vertebrates. Curr. Biol. 2015, 25, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Spandidos, A.; Wang, X.; Wang, H.; Seed, B. PrimerBank: A resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010, 38, D792–D799. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinf. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wüllner, U.; Isenmann, S.; Gleichmann, M.; Klockgether, T.; Bähr, M. Expression of neurotrophins and neurotrophin receptors in the cerebellum of mutant weaver and lurcher mice. Brain Res. Dev. Brain Res. 1998, 110, 1–6. [Google Scholar] [CrossRef]








| Gene Name | NCBI GI Number | Primer Sequence (5’ = Forward; 3’ = Reverse) | Application and Primer Bank ID | Amplicon Size (bp) |
|---|---|---|---|---|
| TrkA (NTRK1) | 568922048 | 5’: CAGTCTGATGACTTCGTTGATGC 3’: CTCTTCACGATGGTTAGGCTTC 5’: AAGGCGGGCGCCGCCGCGAT 3’: TCTCAACTCCCCCAGGCCCT | qPCR 169234625c1 | 243 |
Probe for RISH | 300 | |||
| TrkB (NTRK2) | 68215969 | 5’: CTGGGGCTTATGCCTGCTG 3’: AGGCTCAGTACACCAAATCCTA 5’: GACAGGCTCAGCCTCTGGTA 3’: TTGAGCCACATGATGTCGCA | qPCR 6679150a1 | 100 |
Probe for RISH | 630 | |||
| TrkC (NTRK3) | 755520797 | 5’: CTGAGTGCTACAATCTAAGCCC 3’: CACACCCCATAGAACTTGACAAT 5’: GAATAGTCTCATGGCATATC 3’: CATCCAATGCAGACACTAGA | qPCR 33413412a1 | 157 |
Probe for RISH | 210 | |||
| p75NTR (TNFR) | 70794802 | 5’: CTAGGGGTGTCCTTTGGAGGT 3’: CAGGGTTCACACACGGTCT 5’: TGCAATTAGTAGAAGGACCCCACC 3’: TACACAGGATGCAAAGGGGA | qPCR 15082265a1 | 140 |
Probe for RISH | 264 | |||
| NGF | 162951830 | 5’: TGATCGGCGTACAGGCAGA 3’: GCTGAAGTTTAGTCCAGTGGG 5’: AAACTTCAGCATTCCCTTGA 3’: CCTGTTGAAAGGGATTGTAC | qPCR 162951830c1 | 87 |
Probe for RISH | 231 | |||
| BDNF | 34328441 | 5’: TCATACTTCGGTTGCATGAAGG 3’: AGACCTCTCGAACCTGCCC 5’: GAAAGTCCCGGTATCCAAAG 3’: CCAGCCAATTCTCTTTTT | qPCR and RISH 34328442a1 | 137 |
| Probe for RISH | 181 | |||
| NT-3 | 568941025 | 5’: GGAGTTTGCCGGAAGACTCTC 3’: GGGTGCTCTGGTAATTTTCCTTA 5’: TACAGGTGAACAAGGTGATG 3’: CCTGCTCTGGTTCCCTGGGT | qPCR 6679144a1 | 117 |
Probe for RISH | 240 | |||
| NT-4 | 755521409 | 5’: TGAGCTGGCAGTATGCGAC 3’: CAGCGCGTCTCGAAGAAGT 5’: CTCTTCCTGCTGGAGGCCGG 3’: GCTTTCGGCCTTGCAGCGCGT | qPCR 30353913a1 | 147 |
Probe for RISH | 261 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlMatrouk, A.; Lemons, K.; Ogura, T.; Luo, W.; Wilson, C.; Lin, W. Chemical Exposure-Induced Changes in the Expression of Neurotrophins and Their Receptors in the Main Olfactory System of Mice Lacking TRPM5-Expressing Microvillous Cells. Int. J. Mol. Sci. 2018, 19, 2939. https://doi.org/10.3390/ijms19102939
AlMatrouk A, Lemons K, Ogura T, Luo W, Wilson C, Lin W. Chemical Exposure-Induced Changes in the Expression of Neurotrophins and Their Receptors in the Main Olfactory System of Mice Lacking TRPM5-Expressing Microvillous Cells. International Journal of Molecular Sciences. 2018; 19(10):2939. https://doi.org/10.3390/ijms19102939
Chicago/Turabian StyleAlMatrouk, Abdullah, Kayla Lemons, Tatsuya Ogura, Wangmei Luo, Chantel Wilson, and Weihong Lin. 2018. "Chemical Exposure-Induced Changes in the Expression of Neurotrophins and Their Receptors in the Main Olfactory System of Mice Lacking TRPM5-Expressing Microvillous Cells" International Journal of Molecular Sciences 19, no. 10: 2939. https://doi.org/10.3390/ijms19102939
APA StyleAlMatrouk, A., Lemons, K., Ogura, T., Luo, W., Wilson, C., & Lin, W. (2018). Chemical Exposure-Induced Changes in the Expression of Neurotrophins and Their Receptors in the Main Olfactory System of Mice Lacking TRPM5-Expressing Microvillous Cells. International Journal of Molecular Sciences, 19(10), 2939. https://doi.org/10.3390/ijms19102939