Expression of the O-Glycosylation Enzyme GalNAc-T3 in the Equatorial Segment Correlates with the Quality of Spermatozoa
Abstract
:1. Introduction
2. Results
2.1. GalNAc-T3 Is Expressed in the Equatorial Segment of the Sperm Head and Is Highly Expressed in Motile Sperm Cells
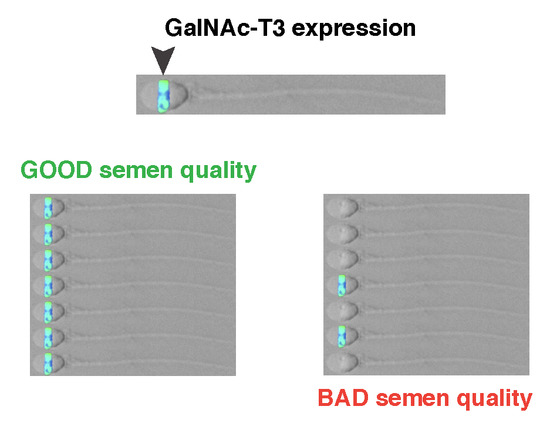
2.2. Association between GalNAc-T3 Expression and Classical Semen Parameters
3. Discussion
4. Materials and Methods
4.1. Ethical Considerations
4.2. Semen Samples
4.3. Swim-up, Capacitation and Acrosome Reaction
4.4. Immunofluorescence (IF)
4.5. Immunocytochemistry (ICC)
4.6. Evaluation of GalNAc-T3 Staining
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Buck Louis, G.M.; Toppari, J.; Andersson, A.-M.; Eisenberg, M.L.; Jensen, T.K.; Jorgensen, N.; Swan, S.H.; Sapra, K.J.; et al. Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility. Physiol. Rev. 2016, 96, 55–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, T.J.; Vitrikas, K.R. Evaluation and treatment of infertility. Am. Fam. Physician 2015, 91, 308–314. [Google Scholar] [PubMed]
- Bonde, J.; Ernst, E.; Jensen, T.; Hjollund, N.; Kolstad, H.; Henriksen, T.; Scheike, T.; Giwercman, A.; Olsen, J.; Skakkebaek, N. Relation between semen quality and fertility: A population-based study of 430 first-pregnancy planners. Lancet 1998, 352, 1172–1177. [Google Scholar] [CrossRef]
- Slama, R.; Eustache, F.; Ducot, B.; Jensen, T.K.; Jorgensen, N.; Horte, A.; Irvine, S.; Suominen, J.; Andersen, A.G.; Auger, J.; et al. Time to pregnancy and semen parameters: A cross-sectional study among fertile couples from four European cities. Hum. Reprod. 2002, 17, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M.; Sundaram, R.; Schisterman, E.F.; Sweeney, A.; Lynch, C.D.; Kim, S.; Maisog, J.M.; Gore-Langton, R.; Eisenberg, M.L.; Chen, Z. Semen quality and time to pregnancy: The longitudinal investigation of fertility and the environment study. Fertil. Steril. 2014, 101, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Guzick, D.S.; Overstreet, J.W.; Factor-Litvak, P.; Brazil, C.K.; Nakajima, S.T.; Coutifaris, C.; Carson, S.A.; Cisneros, P.; Steinkampf, M.P.; Hill, J.A.; et al. Sperm morphology, motility, and concentration in fertile and infertile men. N. Engl. J. Med. 2001, 345, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Björndahl, L. What is normal semen quality? On the use and abuse of reference limits for the interpretation of semen analysis results. Hum. Fertil. 2011, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, S.; Paasch, U.; Glander, H.J.; Anderegg, U. Mature human spermatozoa do not transcribe novel RNA. Andrologia 2005, 37, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, X.; Wang, Z.; Wang, D. Is transcription in sperm stationary or dynamic? J. Reprod. Dev. 2017, 63, 439–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosken, D.J.; Hodgson, D.J. Why do sperm carry RNA? Relatedness, conflict, and control. Trends Ecol. Evol. 2014, 29, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Trevino, C.L. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef] [PubMed]
- Brohi, R.D.; Huo, L.J. Posttranslational modifications in spermatozoa and effects on male fertility and sperm viability. OMICS 2017, 21, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Tecle, E.; Gagneux, P. Sugar-coated sperm: Unraveling the functions of the mammalian sperm glycocalyx. Mol. Reprod. Dev. 2015, 82, 635–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheon, Y.P.; Kim, C.H. Impact of glycosylation on the unimpaired functions of the sperm. Clin. Exp. Reprod. Med. 2015, 42, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P. Sperm-egg interaction. Annu. Rev. Physiol. 2012, 74, 477–502. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.J.; Clausen, H.; Bard, F. Location, location, location: New insights into O-GalNAc protein glycosylation. Trends Cell Biol. 2011, 21, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Goth, C.K.; Vakhrushev, S.Y.; Joshi, H.J.; Clausen, H.; Schjoldager, K.T. Fine-tuning limited proteolysis: A major role for regulated site-specific O-Glycosylation. Trends Biochem. Sci. 2018, 43, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 2012, 22, 736–756. [Google Scholar] [CrossRef] [PubMed]
- Revoredo, L.; Wang, S.; Bennett, E.P.; Clausen, H.; Moremen, K.W.; Jarvis, D.L.; Ten Hagen, K.G.; Tabak, L.A.; Gerken, T.A. Mucin-type O-glycosylation is controlled by short- and long-range glycopeptide substrate recognition that varies among members of the polypeptide GalNAc transferase family. Glycobiology 2016, 26, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Clausen, H.; Bennett, E.P. A family of UDP-GalNAc: Polypeptide N-acetylgalactosaminyl-transferases control the initiation of mucin-type O-linked glycosylation. Glycobiology 1996, 6, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.P.; Hassan, H.; Clausen, H. cDNA cloning and expression of a novel human UDP-N-acetyl-α-d-galactosamine. Polypeptide N-acetylgalactosaminyltransferase, GalNAc-t3. J. Biol. Chem. 1996, 271, 17006–17012. [Google Scholar] [CrossRef] [PubMed]
- Rajpert-De Meyts, E.; Poll, S.; Goukasian, I.; Jeanneau, C.; Herlihy, A.; Bennett, E.; Skakkebaek, N.; Clausen, H.; Giwercman, A.; Mandel, U. Changes in the profile of simple mucin-type O-glycans and polypeptide GalNAc-transferases in human testis and testicular neoplasms are associated with germ cell maturation and tumour differentiation. Virchows Arch. 2007, 451, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.P.; Hassan, H.; Mandel, U.; Hollingsworth, M.A.; Akisawa, N.; Ikematsu, Y.; Merkx, G.; van Kessel, A.G.; Olofsson, S.; Clausen, H. Cloning and characterization of a close homologue of human UDP-N-acetyl-α-d-galactosamine: Polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy. J. Biol. Chem. 1999, 274, 25362–25370. [Google Scholar] [CrossRef] [PubMed]
- Damsgaard, J.; Joensen, U.N.; Carlsen, E.; Erenpreiss, J.; Jensen, M.B.; Matulevicius, V.; Zilaitiene, B.; Olesen, I.A.; Perheentupa, A.; Punab, M.; et al. Varicocele is associated with impaired semen quality and reproductive hormone levels: A study of 7035 healthy young men from six European countries. Eur. Urol. 2016, 70, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Kotula-Balak, M.; Tworzydlo, W.; Pochec, E.; Zarzycka, M.; Bilinska, B. Octylphenol induces changes in glycosylation pattern, calcium level and ultrastructure of bank vole spermatozoa in vitro. Toxicol. In Vitro 2015, 29, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Egeberg Palme, D.L.; Rehfeld, A.; Bang, A.K.; Nikolova, K.A.; Kjærulff, S.; Petersen, M.R.; Jeppesen, J.V.; Glensbjerg, M.; Juul, A.; Skakkebæk, N.E.; et al. Viable acrosome-intact human spermatozoa in the ejaculate as a marker of semen quality and fertility status. Hum. Reprod. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.M.; Moore, H.D.; Franklin, L.E. Significance of the equatorial segment of the acrosome of the spermatozoon in eutherian mammals. Exp. Cell Res. 1979, 119, 119–126. [Google Scholar] [CrossRef]
- Ichikawa, S.; Sorenson, A.H.; Austin, A.M.; Mackenzie, D.S.; Fritz, T.A.; Moh, A.; Hui, S.L.; Econs, M.J. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology 2009, 150, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Szczygiel, M.; Kurpisz, M. Teratozoospermia and its effect on male fertility potential. Andrologia 1999, 31, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, W.; Luo, Y.; Long, X.; Sun, X. Predictive value of semen parameters in in vitro fertilisation pregnancy outcome. Andrologia 2009, 41, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ramalho-Santos, J.; Moreno, R.D.; Wessel, G.M.; Chan, E.K.; Schatten, G. Membrane trafficking machinery components associated with the mammalian acrosome during spermiogenesis. Exp. Cell Res. 2001, 267, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.; Joensen, U.N.; Jensen, T.K.; Jensen, M.B.; Almstrup, K.; Olesen, I.A.; Juul, A.; Andersson, A.-M.; Carlsen, E.; Petersen, J.H.; et al. Human semen quality in the new millennium: A prospective cross-sectional population-based study of 4867 men. BMJ Open 2012, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menkveld, R.; Wong, W.Y.; Lombard, C.J.; Wetzels, A.M.; Thomas, C.M.; Merkus, H.M.; Steegers-Theunissen, R.P. Semen parameters, including WHO and strict criteria morphology, in a fertile and subfertile population: An effort towards standardization of in-vivo thresholds. Hum. Reprod. 2001, 16, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, D.L.; Kjaerulff, S.; Hansen, C.; Petersen, J.H.; Glensbjerg, M.; Skakkebaek, N.E.; Jørgensen, N.; Almstrup, K. Image cytometer method for automated assessment of human spermatozoa concentration. Andrology 2013, 1, 615–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egeberg Palme, D.L.; Johannsen, T.H.; Petersen, J.H.; Skakkebæk, N.E.; Juul, A.; Jørgensen, N.; Almstrup, K. Validation of image cytometry for sperm concentration measurement: Comparison with manual counting of 4010 human semen samples. Clin. Chim. Acta 2017, 468, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Mandel, U.; Hassan, H.; Therkildsen, M.H.; Rygaard, J.; Jakobsen, M.H.; Juhl, B.R.; Dabelsteen, E.; Clausen, H. Expression of polypeptide GalNAc-transferases in stratified epithelia and squamous cell carcinomas: Immunohistological evaluation using monoclonal antibodies to three members of the GalNAc-transferase family. Glycobiology 1999, 9, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, Y.; Joshi, H.J.; Yang, Z.; Gomes, C.; Chen, Y.H.; Lorenzetti, F.C.; Furukawa, S.; Schjoldager, K.T.; Hansen, L.; Clausen, H.; et al. A validated gRNA library for CRISPR/Cas9 targeting of the human glycosyltransferase genome. Glycobiology 2018, 28, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Schjoldager, K.T.; Joshi, H.J.; Kong, Y.; Goth, C.K.; King, S.L.; Wandall, H.H.; Bennett, E.P.; Vakhrushev, S.Y.; Clausen, H. Deconstruction of O-glycosylation—GalNAc-T isoforms direct distinct subsets of the O-glycoproteome. EMBO Rep. 2015, 16, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Böhm, C.M.; Mulder, M.C.; Zennadi, R.; Notter, M.; Schmitt-Gräff, A.; Finn, O.J.; Taylor-Papadimitriou, J.; Stein, H.; Clausen, H.; Riecken, E.O.; et al. Carbohydrate recognition on MUC1-expressing targets enhances cytotoxicity of a T cell subpopulation. Scand. J. Immunol. 1997, 46, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mandel, U.; Petersen, O.W.; Sørensen, H.; Vedtofte, P.; Hakomori, S.; Clausen, H.; Dabelsteen, E. Simple mucin-type carbohydrates in oral stratified squamous and salivary gland epithelia. J. Investig. Dermatol. 1991, 97, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Steentoft, C.; Vakhrushev, S.Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Kong, Y.; Bennett, E.P.; Mandel, U.; Wandall, H.; Levery, S.B.; Clausen, H. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat Methods 2011, 8, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- The Comprehensive R Archive Network. Available online: https://cran.r-project.org/ (accessed on 5 September 2018).





| Semen Parameter | N | Mean | Median | Min | Q1 | Q3 | Max |
|---|---|---|---|---|---|---|---|
| Volume (mL) | 206 | 3.8 | 3.7 | 0.9 | 2.8 | 4.7 | 7.4 |
| Concentration (mill/mL) | 206 | 39.7 | 28.0 | 0.0 | 9.6 | 58.0 | 217.0 |
| Total count (mill) | 206 | 148.8 | 106.1 | 0.0 | 37.6 | 221.5 | 1215.2 |
| Progressive motile (%) | 206 | 44.9 | 48.0 | 0.0 | 28.0 | 63.0 | 85.0 |
| Morphological normal forms (%) | 205 | 5.3 | 4.5 | 0.0 | 2.0 | 8.0 | 23.5 |
| GalNAc-T3 positive spermatozoa (%) | 206 | 48.4 | 55.5 | 0.0 | 17.4 | 76.5 | 94.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nygaard, M.B.; Herlihy, A.S.; Jeanneau, C.; Nielsen, J.E.; Bennett, E.P.; Jørgensen, N.; Clausen, H.; Mandel, U.; Rajpert-De Meyts, E.; Almstrup, K. Expression of the O-Glycosylation Enzyme GalNAc-T3 in the Equatorial Segment Correlates with the Quality of Spermatozoa. Int. J. Mol. Sci. 2018, 19, 2949. https://doi.org/10.3390/ijms19102949
Nygaard MB, Herlihy AS, Jeanneau C, Nielsen JE, Bennett EP, Jørgensen N, Clausen H, Mandel U, Rajpert-De Meyts E, Almstrup K. Expression of the O-Glycosylation Enzyme GalNAc-T3 in the Equatorial Segment Correlates with the Quality of Spermatozoa. International Journal of Molecular Sciences. 2018; 19(10):2949. https://doi.org/10.3390/ijms19102949
Chicago/Turabian StyleNygaard, Marie B., Amy S. Herlihy, Charlotte Jeanneau, John E. Nielsen, Eric Paul Bennett, Niels Jørgensen, Henrik Clausen, Ulla Mandel, Ewa Rajpert-De Meyts, and Kristian Almstrup. 2018. "Expression of the O-Glycosylation Enzyme GalNAc-T3 in the Equatorial Segment Correlates with the Quality of Spermatozoa" International Journal of Molecular Sciences 19, no. 10: 2949. https://doi.org/10.3390/ijms19102949
APA StyleNygaard, M. B., Herlihy, A. S., Jeanneau, C., Nielsen, J. E., Bennett, E. P., Jørgensen, N., Clausen, H., Mandel, U., Rajpert-De Meyts, E., & Almstrup, K. (2018). Expression of the O-Glycosylation Enzyme GalNAc-T3 in the Equatorial Segment Correlates with the Quality of Spermatozoa. International Journal of Molecular Sciences, 19(10), 2949. https://doi.org/10.3390/ijms19102949