Anthocyanins Extracted from Oryza sativa L. Prevent Fluorouracil-Induced Nuclear Factor-κB Activation in Oral Mucositis: In Vitro and In Vivo Studies
Abstract
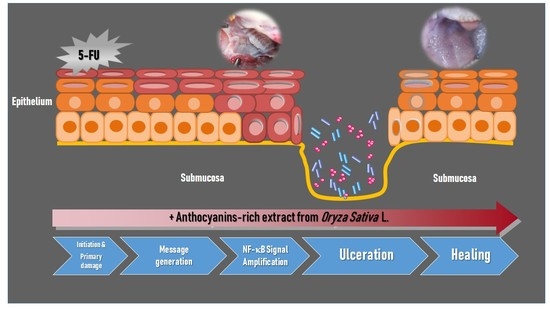
:1. Introduction
2. Results
2.1. Identification of Anthocyanins (ANT) in Oryza sativa L.
2.2. General Conditions and Body Weight of Subjects
2.3. Oral Mucositis Lesions Are Attenuated in ANT-Pretreated Rats
2.4. Effect of ANT-Pretreatment on Histopathological Aspects of 5-FU-Induced Oral Mucositis
2.5. ANT Suppressed 5-FU-Induced Transcription Factor NF-κB p50 and p65 Activation in Oral Mucositis
2.6. Pretreatment with ANT for 1000 mg/kg Blocks HMGB1 Levels in 5-FU-Induced Oral Mucositis
2.7. ANT Protect 5-FU-induced Oral Keratinocyte Cell Growth Suppression
2.8. ANT Suppresses NF-κB p50 and p65 Levels in Nuclear Fraction of Oral Keratinocytes
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Plant Material and Extraction
4.3. Quantification of ANT by UV-Vis Spectroscopy and Antioxidant Activities
4.4. Animal Model
4.5. Induction of Experimental Oral Mucositis
4.6. Animals and Study Design
4.7. Macroscopic Analysis of Buccal Mucosae
4.8. Histopathological Analysis
4.9. Immunohistochemistry for p50 and p65 NF-κB and HMGB1
4.10. Cell Culture Conditions
4.11. Cell Viability Test
4.12. Preparation of Nucleic/Cytosolic Fractions
4.13. Western Blot Analysis
4.14. HMGB1 Measurement by Enzyme-Linked Immunosorbent Assay
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karthaus, R.C.; Ganser, A. Prophylaxis and treatment of chemo-and radiotherapy-induced oral mucositis. Bone Marrow Transplant. 1999, 24, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. Mucositis as a biological process: A new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1998, 34, 39–43. [Google Scholar] [CrossRef]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Yeoh, A.S.; Gibson, R.J.; Sonis, S.T. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: Pathobiology, animal models and cytotoxic drugs. Cancer Treat. Rev. 2007, 33, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. Oral mucositis. Anti-Cancer Drugs 2011, 22, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M. Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered? Cancer Chemother. Pharmcol. 2009, 63, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.E. New strategies for management of oral mucositis in cancer patients. J. Support. Oncol. 2006, 4, 10–13. [Google Scholar]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, G.M.; Awde, J.D.; Ghandi, H.; Vincent, M.; Kocha, W.I. Risk factors associated with mucositis in cancer patients receiving 5-fluorouracil. Oral Oncol. 1998, 34, 484–490. [Google Scholar] [CrossRef]
- Tomioka, M.T.; Kataoka, Y.; Ekyalongo, R.C.; Funakoshi, Y.; Imai, Y.; Kiyota, N.; Fujiwara, Y.; Minami, H. Inhibition of the mTOR/S6K signal is necessary to enhance fluorouracil-induced apoptosis in gastric cancer cells with HER2 amplification. Int. J. Oncol. 2012, 41, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.S.; Park, E.Y.; Shin, S.M. 5-Fluorouracil inhibits cell migration by induction of Sestrin2 in colon cancer cells. Arch. Pharm. Res. 2017, 40, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M. Nuclear factor-κB (NF-κB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol. 2007, 43, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, H.; Hashemi, S.R.; Seow, H.F. 5-Fluorouracil induce the expression of TLR4 on HCT116 colorectal cancer cell line expressing different variants of TLR4. Iran. J. Pharm. Res. 2013, 12, 453–460. [Google Scholar] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Chung, E.Y.; Jang, H.Y.; Hong, O.Y.; Chae, H.S.; Jeong, Y.J. Anti-cancer effect of cyanidin-3-glucoside from mulberry via caspase-3 cleavage and DNA fragmentation in vitro and in vivo. Anti-Cancer Agents Med. Chem. 2017, 17, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, Y.M.; Chung, J.I.; Shin, S.C.; Jeong, Y.K.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from black soybean seed coats stimulate wound healing in fibroblasts and keratinocytes and prevent inflammation in endothelial cells. Food Chem. Toxicol. 2009, 47, 2806–2812. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, R.L.; Laake, P.; Paur, I.; Bøhn, S.K.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.S.; Dugo, P.; Paola, R.D.; Mondello, L.; Genovese, T.; Morabito, D.; Dugo, G.; Sautebin, L.; Caputi, A. Protective effects of anthocyanins from blackberry in a rat model of acute lung inflammation. Free Radic. Res. 2003, 37, 891–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, T.; Yuan, Y.; Wu, S.-C.; Hong, D. Beneficial effects of anthocyanins from red cabbage (Brassica oleracea L. var capitata L.) administration to prevent irinotecan-induced mucositis. J. Funct. Foods 2017, 32, 9–17. [Google Scholar]
- Hou, Z.H.; Qin, P.Y.; Ren, G.X. Effect of anthocyanin-rich extract from black rice (Oryza sativa L. Japonica) on chronically alcohol-induced liver damage in rats. J. Agric. Food Chem. 2010, 58, 3191–3196. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Yu, B.; Yo, X.P.; Yi, L.; Chen, C.Y.; Mi, M.T. Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr. Cancer 2010, 62, 1128–1136. [Google Scholar]
- Duncan, M.; Grant, G. Oral and intestinal mucositis-causes and possible treatments. Aliment. Pharmacol. Ther. 2003, 18, 853–874. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Ho, T.Y.; Lin, H.; Liang, J.A.; Huang, H.C.; Li, C.C. 5-Fluorouracil induced intestinal mucositis via nuclear factor-kappaB activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS ONE 2012, 7, e31808. [Google Scholar]
- Cottone, L.; Capobianco, A.; Gualteroni, C.; Perrotta, C.; Bianchi, M.E.; Rovere-Querini, P.; Manfredi, A.A. 5-Fluorouracil causes leukocytes attraction in the peritoneal cavity by activating autophagy and HMGB1 release in colon carcinoma cells. Int. J. Cancer 2015, 136, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Chaichalotornkul, S.; Nararatwanchai, T.; Narkpinit, S.; Dararat, P.; Kikuchi, K.; Maruyama, I. Secondhand smoke exposure-induced nucleocytoplasmic shuttling of HMGB1 in a rat premature skin aging model. Biochem. Biophys. Res. Commun. 2015, 456, 92–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, O.H.; Yoon, Y.; Magnuson, B.A.; Kim, M.K.; Chun, H.S. Protective effect of anthocyanin-rich extract from bilberry (Vaccinium myrtillus L.) against myelotoxicity induced by 5-fluorouracil. Biofactors 2007, 29, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Plevova, P. Re: Mucositis as a biological process: A new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1999, 35, 225–226. [Google Scholar] [PubMed]
- Miyata, H.; Asanuma, F.; Iwaki, Y.; Kimura, M.; Matsumoto, K. Evaluation of myelotoxicity in dietary restricted rats. J. Toxicol. Pathol. 2009, 22, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Pedro, A.C.; Granato, D.; Rosso, N.D. Extraction of anthocyanins and polyphenols from black rice (Oryza sativa L.) by modeling and assessing their reversibility and stability. Food Chem. 2016, 191, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Sompong, R.; Siebenhandl-Ehn, S.; Linsberger-Martin, G.; Berghofer, E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011, 124, 132–140. [Google Scholar] [CrossRef]
- Sonis, T.C.; Shklar, G.; Jenson, J.; Florine, D. An animal model for mucositis induced by cancer chemotherapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1990, 69, 437–443. [Google Scholar] [CrossRef]
- Sonis, S.T. New thoughts on the initiation of mucositis. Oral Dis. 2010, 16, 597–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pahl, H.L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswal, B.M. Current trends in the management of oral mucositis related to cancer treatment. Malays. J. Med. Sci. 2008, 15, 4–13. [Google Scholar] [PubMed]
- Schenck, K.; Schreurs, O.; Hayashi, K.; Helgeland, K. The role of nerve growth factor (NGF) and its precursor forms in oral wound healing. Int. J. Mol. Sci. 2017, 11, E386. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.B.; de Araújo, A.A.; Araújo Júnior, R.F.; Brito, G.A.C.; Leitão, R.C.; Barbosa, M.M.; Garcia, V.B.; Medeiros, A.C.; Medeiros, C.A.C.X. Protective effect of dexamethasone on 5-FU-Induced oral mucositis in hamsters. PLoS ONE 2017, 23, e0186511. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.F. Oral mucositis, dysfunction, and distress in patients undergoing cancer therapy. J. Clin. Nurs. 2007, 16, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Kaneko, A.; Matsumoto, C.; Miyagi, C.; Ohbuchi, K.; Mizuhara, Y. Multitargeted effects of hangeshashinto for treatment of chemotherapy-induced oral mucositis on inducible prostaglandin E2 production in human oral keratinocytes. Integr. Cancer Ther. 2014, 13, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Sarikaphuti, A.; Nararatwanchai, T.; Hashiguchi, T.; Ito, T.; Thaworanunta, S.; Kikuchi, K.; Oyama, Y.; Maruyama, I.; Tancharoen, S. Preventive effects of Morus alba L. anthocyanins on diabetes in Zucker diabetic fatty rats. Exp. Ther. Med. 2013, 6, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.G.; Cunha, F.Q.; Rebouc, C.G.; Falcão, B.A.; Augusto, R.F.; Souza, M.L.P.; Leitão, B.T.; Ribeiro, R.A. Effects of the tumour necrosis factor-α inhibitors pentoxifylline and thalidomide in short-term experimental oral mucositis in hamsters. Eur. J. Oral Sci. 2005, 113, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Tancharoen, S.; Binita, S.; Nagasato, T.; Kikuchi, K.; Nawa, Y.; Dararat, P.; Yamamoto, M.; Narkpinit, S.; Maruyama, I. HMGB1 promotes intraoral palatal wound healing through RAGE-dependent mechanisms. Int. J. Mol. Sci. 2016, 17, E1961. [Google Scholar] [CrossRef] [PubMed]
- Miot, H.A.; Brianezi, G. Morphometric analysis of dermal collagen by color clusters segmentation. An. Bras. Dermatol. 2010, 85, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Tancharoen, S.; Tengrungsun, T.; Suddhasthira, T.; Kikuchi, K.; Vechvongvan, N.; Tokuda, M.; Maruyama, I. Overexpression of receptor for advanced glycation end products and high-mobility group box 1 in human dental pulp inflammation. Mediat. Inflamm. 2014, 2014, 754069. [Google Scholar] [CrossRef] [PubMed]








| Compound No. * | RT (min) | MS, M+ (m/z) | MS/MS (m/z) | Assignment ** |
|---|---|---|---|---|
| 1 | 31.0 | 449.1 | 287 | Cyanidin-3-glucoside |
| 2 | 37.0 | 463.1 | 301 | Pelargonidin-3-glucoside |
| Parameter | Crude Extract | Purified Extract |
|---|---|---|
| Total antioxidant capacity (mM/g) | 1.07 ± 0.06 | 1.660 ± 0.297 * |
| FRAP (mM Fe (II) equivalents/gram of fresh weight) | 27.23 ± 1.00 | 28.06 ± 3.66 |
| Total phenol content (mM Gallic acid equivalent/gFW) | 181.73 ± 12.79 | 193.4 ± 6.71 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tancharoen, S.; Shakya, P.; Narkpinit, S.; Dararat, P.; Kikuchi, K. Anthocyanins Extracted from Oryza sativa L. Prevent Fluorouracil-Induced Nuclear Factor-κB Activation in Oral Mucositis: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018, 19, 2981. https://doi.org/10.3390/ijms19102981
Tancharoen S, Shakya P, Narkpinit S, Dararat P, Kikuchi K. Anthocyanins Extracted from Oryza sativa L. Prevent Fluorouracil-Induced Nuclear Factor-κB Activation in Oral Mucositis: In Vitro and In Vivo Studies. International Journal of Molecular Sciences. 2018; 19(10):2981. https://doi.org/10.3390/ijms19102981
Chicago/Turabian StyleTancharoen, Salunya, Prana Shakya, Somphong Narkpinit, Pornpen Dararat, and Kiyoshi Kikuchi. 2018. "Anthocyanins Extracted from Oryza sativa L. Prevent Fluorouracil-Induced Nuclear Factor-κB Activation in Oral Mucositis: In Vitro and In Vivo Studies" International Journal of Molecular Sciences 19, no. 10: 2981. https://doi.org/10.3390/ijms19102981
APA StyleTancharoen, S., Shakya, P., Narkpinit, S., Dararat, P., & Kikuchi, K. (2018). Anthocyanins Extracted from Oryza sativa L. Prevent Fluorouracil-Induced Nuclear Factor-κB Activation in Oral Mucositis: In Vitro and In Vivo Studies. International Journal of Molecular Sciences, 19(10), 2981. https://doi.org/10.3390/ijms19102981