Beneficial Effects of Deoxyshikonin on Delayed Wound Healing in Diabetic Mice
Abstract
:1. Introduction
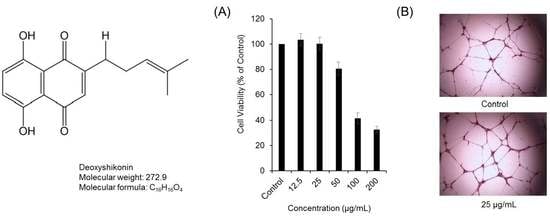
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Cytotoxicity and Proliferation Assay
3.4. DPPH Assay
3.5. Tube-Formation Assay in HUVECs
3.6. Western Blotting Analysis
3.7. Semi-Quantitative Reverse-Transcriptase PCR (RT-PCR)
3.8. Assessment of Wound Healing in Streptozotocin-Induced Diabetes Mice
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Eming, S.A.; Brachvogel, B.; Odorisio, T.; Koch, M. Regulation of angiogenesis: Wound healing as a model. Prog. Histochem. Cytochem. 2007, 42, 115–170. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Yamori, T.; Kobayashi, M.; Duan, H. Antiproliferative and antiangiogenic activities of smenospongine, a marine sponge sesquiterpene aminoquinone. Mar. Drugs 2011, 9, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Oklu, R.; Walker, T.G.; Wicky, S.; Hesketh, R. Angiogenesis and current antiangiogenic strategies for the treatment of cancer. J. Vasc. Interv. Radiol. 2010, 21, 1791–1805. [Google Scholar] [CrossRef] [PubMed]
- Risau, W. Differentiation of endothelium. FASEB J. 1995, 9, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kwon, Y.G. Roles of YAP in mediating endothelial cell junctional stability and vascular remodeling. BMB Rep. 2015, 48, 429–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Ii, M.; Takenaka, H.; Asai, J.; Ibusuki, K. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ. Res. 2006, 98, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Schatteman, G.C.; Hanlon, H.D.; Jiao, C.; Dodds, S.G. Loodderived angioblasts accelerate blood-flow restoration in diabetic mice. J. Clin. Investig. 2000, 106, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Galiano, R.D.; Capla, J.M.; Kalka, C. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002, 106, 2781–2786. [Google Scholar] [CrossRef] [PubMed]
- Keswani, S.G.; Katz, A.B.; Lim, F.Y.; Zoltick, P. Adenoviral mediated gene transfer of PDGF-B enhances wound healing in type I and type II diabetic wounds. Wound Repair Regen. 2004, 12, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Loomans, C.J.; de Koning, E.J.; Staal, F.J.; Rookmaaker, M.B. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004, 53, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: New insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Wang, G.G.; Li, W.; Jiang, Y.X. Heme Oxygenase-1 Promotes Delayed Wound Healing in Diabetic Rats. J. Diabetes Res. 2016, 2016, 9726503. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kim, G.J. Effects of Gamijaungo on the burn mice model and the study of hematologic, pathologic and molecular mechanism. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2015, 28, 53–67. (In Korean) [Google Scholar] [CrossRef] [Green Version]
- Lu, P.J.; Yang, C.; Lin, C.N.; Li, C.F. Shiunko and acetylshikonin promote reepithelialization, angiogenesis, and granulation tissue formation in wounded skin. Am. J. Chin. Med. 2008, 36, 115–123. [Google Scholar]
- Jeon, J.H.; Hong, S.U. The effects of Jawoongo on UVB Damage to Skin and Photoaging. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2007, 20, 130–144. (In Korean) [Google Scholar]
- Prangsaengtong, O.; Park, J.Y.; Inujima, A.; Igarashi, Y. Enhancement of Lymphangiogenesis In Vitro via the Regulations of HIF-1α Expression and Nuclear Translocation by Deoxyshikonin. Evid. Based Complement. Altern. Med. 2013, 2013, 148297. [Google Scholar] [CrossRef] [PubMed]
- Komi, Y.; Suzuki, Y.; Shimamura, M.; Kajimoto, S. Mechanism of inhibition of tumor angiogenesis by beta-hydroxyisovalerylshikonin. Cancer Sci. 2009, 100, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kwak, J.H.; Kang, K.S.; Jung, E.B. Wound healing effects of deoxyshikonin isolated from Jawoongo: In vitro and in vivo studies. J. Ethnopharmacol. 2017, 199, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Lee, S.H.; Shin, M.J.; Kim, K. PEP-1-FK506BP inhibits alkali burn-induced corneal inflammation on the rat model of corneal alkali injury. BMB Rep. 2015, 48, 618–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Hylands, P.J.; Mensah, A.Y.; Hensel, A. In vitro tests and ethnopharmacological investigations: Wound healing as an example. J. Ethnopharmacol. 2005, 100, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Pattreeya, T.; Teeratad, S.; Sukanlaya, L.; Chatchai, W. Wound healing activity of Curcuma zedoaroides. Songklanakarin J. Sci. Technol. 2016, 38, 621–630. [Google Scholar]
- Xu, Y.; Xu, X.; Gao, X.; Chen, H. Shikonin suppresses IL-17-induced VEGF expression via blockage of JAK2/STAT3 pathway. Int. Immunopharmacol. 2014, 19, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Hisa, T.; Kimura, Y.; Takada, K.; Suzuki, F. Shikonin, an ingredient of Lithospermum erythrorhizon, inhibits angiogenesis in vivo and in vitro. Anticancer Res. 1998, 18, 783–790. [Google Scholar] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S. Growth factors and cytokines in wound healing. Wound Repair Regen. 2009, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, Y.K.; Lee, D.S.; Yoo, J.E. Abietic acid isolated from pine resin (Resina Pini) enhances angiogenesis in HUVECs and accelerates cutaneous wound healing in mice. J. Ethnopharmacol. 2017, 203, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell Commun. Signal. 2016, 10, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.Y.; Choi, P.; Kim, H.K.; Kang, K.S. Increase in apoptotic effect of Panax ginseng by microwave processing in human prostate cancer cells: In vitro and in vivo studies. J. Ginseng Res. 2016, 40, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Le, D.D.; Zhao, B.T.; Ma, E.S.; Min, B.S.; Woo, M.H. Antioxidant Compounds Isolated from the Roots of Phlomis umbrosa Turcz. Nat. Prod. Sci. 2018, 24, 119–124. [Google Scholar] [CrossRef]
- Harhour, A.; Brada, M.; Fauconnier, M.L.; Lognay, G. Chemical Composition and Antioxidant Activity of Algerian Juniperus Phoenicea Essential Oil. Nat. Prod. Sci. 2018, 24, 125–131. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Kim, J.H. Maximization of Extracted Condition of Pro-angiogenic Components in Citrus unshiu Peels using Dimethyl Sulfoxide. Nat. Prod. Sci. 2016, 22, 287–292. [Google Scholar] [CrossRef]
- Roy, A.; Park, H.J.; Jung, H.A.; Choi, J.S. Estragole Exhibits Anti-inflammatory Activity with the Regulation of NF-κB and Nrf-2 Signaling Pathways in LPS-induced RAW 264.7 cells. Nat. Prod. Sci. 2018, 24, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Kim, B.S.; Rhee, K.H. Anti-tumor Activity of Saussurea laniceps against Pancreas Adenocarcinoma. Nat. Prod. Sci. 2017, 23, 281–285. [Google Scholar] [CrossRef]
- Hwang, S.H.; Wang, Z.; Kang, I.J.; Lim, S.S. Synergetic Hepatoprotective Effects of Korean Red Ginseng and Pueraria Radix on the Liver Damaged-Induced by Carbon Tetrachloride (CCl4) in Mice. Nat. Prod. Sci. 2017, 23, 132–138. [Google Scholar] [CrossRef]







| Accession No. | Gene Name | Forward Primer | Reverse Primer |
|---|---|---|---|
| NM_002019.4 | VEGF-A | 5′-GCCTTGCCTTGCTGCTCTA-3′ | 5′-GATGTCCACCAGGGTCTCG-3′ |
| NM_002019.4 | VEGFR-2 | 5′-ACGCCGATTATGTGAGA-3′ | 5′-AGGCAGGAGTTGAGTATGT-3′ |
| NM_001289745.2 | GAPDH | 5′-GTCATCCATGACAACTTTGG-3′ | 5′-GAGCTTGACAAAGTGGTCGT-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.Y.; Shin, M.-S.; Hwang, G.S.; Yamabe, N.; Yoo, J.-E.; Kang, K.S.; Kim, J.-C.; Lee, J.G.; Ham, J.; Lee, H.L. Beneficial Effects of Deoxyshikonin on Delayed Wound Healing in Diabetic Mice. Int. J. Mol. Sci. 2018, 19, 3660. https://doi.org/10.3390/ijms19113660
Park JY, Shin M-S, Hwang GS, Yamabe N, Yoo J-E, Kang KS, Kim J-C, Lee JG, Ham J, Lee HL. Beneficial Effects of Deoxyshikonin on Delayed Wound Healing in Diabetic Mice. International Journal of Molecular Sciences. 2018; 19(11):3660. https://doi.org/10.3390/ijms19113660
Chicago/Turabian StylePark, Jun Yeon, Myoung-Sook Shin, Gwi Seo Hwang, Noriko Yamabe, Jeong-Eun Yoo, Ki Sung Kang, Jin-Chul Kim, Jeong Gun Lee, Jungyeob Ham, and Hye Lim Lee. 2018. "Beneficial Effects of Deoxyshikonin on Delayed Wound Healing in Diabetic Mice" International Journal of Molecular Sciences 19, no. 11: 3660. https://doi.org/10.3390/ijms19113660
APA StylePark, J. Y., Shin, M. -S., Hwang, G. S., Yamabe, N., Yoo, J. -E., Kang, K. S., Kim, J. -C., Lee, J. G., Ham, J., & Lee, H. L. (2018). Beneficial Effects of Deoxyshikonin on Delayed Wound Healing in Diabetic Mice. International Journal of Molecular Sciences, 19(11), 3660. https://doi.org/10.3390/ijms19113660