Glutathione Transferases: Potential Targets to Overcome Chemoresistance in Solid Tumors
Abstract
:1. Mechanisms of Chemoresistance
2. Glutathione Transferases in Cancer
3. Catalytic Role of Glutathione Transferases in Detoxification and/or Bio-Activation of Anti-Cancer Drugs
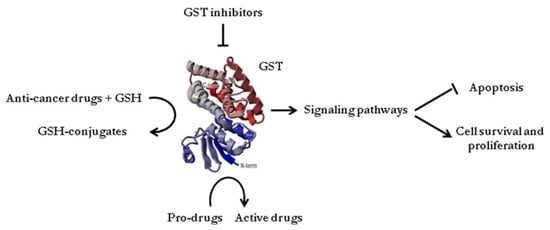
4. Glutathione Transferases in Regulation of Signaling Pathways Involved in Cell Proliferation and Cell Death
- In addition to their catalytic role in detoxification of xenobiotics, GSTs are also involved in the regulation of cellular proliferation and apoptosis by the means of protein-protein interactions with signaling molecules. Regarding GSTM1, the same region of ASK1 seems to be engaged in protein-protein interactions with either GSTM1 or thioredoxin (Trx), suggesting the presence of both GSTM1:ASK1 and ASK1:Trx complexes under unstressed conditions. GSTP1 acts as negative regulator of JNK1, as well as TRAF2. Moreover, GSTP1:TRAF2 interaction prevents ASK1:TRAF2 interaction and, consequently, ASK1 activation. The structural homology between GSTA1 and GSTP1 may explain why GSTA1 can also suppress JNK1 signaling by a similar mechanism. Various types of cell stress can result in the disassociation of GSTs from the signaling molecules. Importantly, redox-sensitive dynamic equilibrium comprises catalytic homodimeric forms of GSTs, as well as its monomeric regulatory forms. ASK1—apoptosis signal—regulating kinase; JNK1-c-Jun N-terminal kinases; TRAF2—tumor necrosis factor receptor-associated factor 2; Trx—thioredoxin;
- GSTO1-1 deglutathionylates some cell death and survival signaling molecules, cytoskeleton and heat shock proteins by forming mixed disulfides with GSH. Specific deglutathionylation by GSTO1-1 leads to the potential protein-specific regulation of protein function. GSTO1-1 also interacts with the ryanodine receptor, RyR1 and promotes calcium release from the endoplasmic reticulum. Increased cytosolic calcium levels activate PYK2 leading to cell proliferation. GSTO1-1 interaction with Akt influences cell survival signaling pathways. RyR1—ryanodine receptor type 1; PYK2—proline-rich tyrosine kinase 2; Akt—protein kinase B.
5. The Role of Glutathione Transferases in Chemoresistance: Potential Targets for Anti-Cancer Agents
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| ANS–etoposide | 4-acetyl-2-nitro-benzenesulfonyl etoposide |
| ANS–DOX | 4-acetyl-2-nitro-benzenesulfonyl doxorubicin |
| ASK1 | apoptosis signal- regulating kinase |
| DHAR | dehydroascorbate reductase |
| DNS–DOX | 2,4-dinitrobenzenesulfonyl doxorubicin |
| DOX | doxorubicin |
| EA | ethacrynic acid |
| EGFR | Epidermal growth factor receptor |
| GSH | glutathione |
| GST | glutathione transferases |
| GSTA | GST Alpha class |
| GSTM | GST Mu class |
| GSTO | GST Omega class |
| GSTP | GST Pi class |
| GSTS | GST Sigma class |
| GSTT | GST Theta class |
| GSTZ | GST Zeta class |
| JNK | c-Jun N-terminal kinases |
| JS-K | O2-(2,4-dinitrophenyl)diazeniumdiolates derivative |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinases |
| M-EA-Pt | ethacraplatin-containing micelles |
| MRP | multi-drug resistant protein |
| NBDHEX | 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio) hexanol |
| NO | Nitric oxide |
| PABA/NO | [O2-{2,4-dinitro-5-[4-(N-methylamino)benzoyloxy]phenyl}1-(N,N-dimethylamino)diazen-1-ium- 1,2-diolate) |
| PG | prostaglandins |
| RyRs | ryanodine receptors |
| SNPs | single nucleotide polymorphisms |
| TER199 | γ-glutamyl cysteinyl phenyl glycyl diethyl ester or TLK199 |
| TLK286 | canfosfamide |
| TLR4 | Toll-like receptor 4 |
| TRAF2 | tumor necrosis factor receptor-associated factor 2 |
| Trx | thioredoxin |
References
- Sau, A.; Pellizzari Tregno, F.; Valentino, F.; Federici, G.; Caccuri, A.M. Glutathione transferases and development of new principles to overcome drug resistance. Arch. Biochem. Biophys. 2010, 500, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Pathania, S.; Bhatia, R.; Baldi, A.; Singh, R.; Rawal, R.K. Drug metabolizing enzymes and their inhibitors’ role in cancer resistance. Biomed. Pharmacother. 2018, 105, 53–65. [Google Scholar] [CrossRef] [PubMed]
- James, M.O.; Jahn, S.C.; Zhong, G.; Smeltz, M.G.; Hu, Z.; Stacpoole, P.W. Therapeutic applications of dichloroacetate and the role of glutathione transferase zeta-1. Pharmacol. Ther. 2017, 170, 166–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pietro, G.; Magno, L.A.V.; Rios-Santos, F. Glutathione S-transferases: An overview in cancer research. Expert Opin. Drug Metab. Toxicol. 2010, 6, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Tew, K.D.; Townsend, D.M. Glutathione-s-transferases as determinants of cell survival and death. Antioxid. Redox Signal. 2012, 17, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Dong, D. Human cytosolic glutathione transferases: Structure, function, and drug discovery. Trends Pharmacol. Sci. 2012, 33, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Activation of alkyl halides by glutathione transferases. Methods Enzymol. 2005, 401, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Kurtovic, S.; Grehn, L.; Karlsson, A.; Hellman, U.; Mannervik, B. Glutathione transferase activity with a novel substrate mimics the activation of the prodrug azathioprine. Anal. Biochem. 2008, 375, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Pulford, D.J. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 445–600. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Takeda, K.; Matsuzawa, A.; Saegusa, K.; Nakano, H.; Gohda, J.; Inoue, J.-I.; Ichijo, H. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J. Biol. Chem. 2005, 280, 37033–37040. [Google Scholar] [CrossRef] [PubMed]
- Tars, K.; Olin, B.; Mannervik, B. Structural basis for featuring of steroid isomerase activity in alpha class glutathione transferases. J. Mol. Biol. 2010, 397, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; McLellan, L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999, 31, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Board, P.G.; Menon, D. Glutathione transferases, regulators of cellular metabolism and physiology. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3267–3288. [Google Scholar] [CrossRef] [PubMed]
- Laborde, E. Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ. 2010, 17, 1373–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIlwain, C.C.; Townsend, D.M.; Tew, K.D. Glutathione S-transferase polymorphisms: Cancer incidence and therapy. Oncogene 2006, 25, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Oakley, A. Glutathione transferases: A structural perspective. Drug Metab. Rev. 2011, 43, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Hollman, A.; Tchounwou, P.; Huang, H.-C. The Association between Gene-Environment Interactions and Diseases Involving the Human GST Superfamily with SNP Variants. Int. J. Environ. Res. Public. Health 2016, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Kellen, E.; Hemelt, M.; Broberg, K.; Golka, K.; Kristensen, V.N.; Hung, R.J.; Matullo, G.; Mittal, R.D.; Porru, S.; Povey, A.; et al. Pooled analysis and meta-analysis of the glutathione S-transferase P1 Ile 105Val polymorphism and bladder cancer: A HuGE-GSEC review. Am. J. Epidemiol. 2007, 165, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Dusinská, M.; Ficek, A.; Horská, A.; Raslová, K.; Petrovská, H.; Vallová, B.; Drlicková, M.; Wood, S.G.; Stupáková, A.; Gasparovic, J.; et al. Glutathione S-transferase polymorphisms influence the level of oxidative DNA damage and antioxidant protection in humans. Mutat. Res. 2001, 482, 47–55. [Google Scholar] [CrossRef]
- Coles, B.F.; Kadlubar, F.F. Human alpha class glutathione S-transferases: Genetic polymorphism, expression, and susceptibility to disease. Methods Enzymol. 2005, 401, 9–42. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Kagawa, T.; Jinno, H.; Hasegawa, T.; Makino, Y.; Seko, Y.; Hanioka, N.; Ando, M. Functional characterization of two variant human GSTO 1-1s (Ala140Asp and Thr217Asn). Biochem. Biophys. Res. Commun. 2003, 301, 516–520. [Google Scholar] [CrossRef]
- Whitbread, A.K.; Tetlow, N.; Eyre, H.J.; Sutherland, G.R.; Board, P.G. Characterization of the human Omega class glutathione transferase genes and associated polymorphisms. Pharmacogenetics 2003, 13, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.; Board, P.G. A Role for Glutathione Transferase Omega 1 (GSTO1-1) in the Glutathionylation Cycle. J. Biol. Chem. 2013, 288, 25769–25779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, B.; Salavaggione, O.E.; Pelleymounter, L.L.; Moon, I.; Eckloff, B.W.; Schaid, D.J.; Wieben, E.D.; Weinshilboum, R.M. Glutathione S-transferase omega 1 and omega 2 pharmacogenomics. Drug Metab. Dispos. 2006, 34, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Zou, F.; Chai, H.; Younkin, C.S.; Miles, R.; Nair, A.A.; Crook, J.E.; Pankratz, V.; Carrasquillo, M.M.; Rowley, C.N.; et al. Glutathione S-transferase omega genes in Alzheimer and Parkinson disease risk, age-at-diagnosis and brain gene expression: An association study with mechanistic implications. Mol. Neurodegener. 2012, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Board, P.; Coggan, M.; Johnston, P.; Ross, V.; Suzuki, T.; Webb, G. Genetic heterogeneity of the human glutathione transferases: A complex of gene families. Pharmacol. Ther. 1990, 48, 357–369. [Google Scholar] [CrossRef]
- Wiencke, J.K.; Pemble, S.; Ketterer, B.; Kelsey, K.T. Gene deletion of glutathione S-transferase theta: Correlation with induced genetic damage and potential role in endogenous mutagenesis. Cancer Epidemiol. Prev. Biomark. 1995, 4, 253–259. [Google Scholar]
- Singh, S. Cytoprotective and regulatory functions of glutathione S-transferases in cancer cell proliferation and cell death. Cancer Chemother. Pharmacol. 2015, 75, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Simic, T.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; Mimic-Oka, J. Glutathione S-transferases in kidney and urinary bladder tumors. Nat. Rev. Urol. 2009, 6, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, C.L.; Peters, W.H.; Scheffer, P.G.; Bouman, A.A.; Boven, E.; Newling, D.W. Glutathione S-transferase activity and subunit composition in transitional cell cancer and mucosa of the human bladder. Urology 1997, 49, 644–651. [Google Scholar] [CrossRef]
- Kaprilian, C.; Horti, M.; Kandilaris, K.; Skolarikos, A.; Trakas, N.; Kastriotis, I.; Deliveliotis, C. Glutathione-S-transferase-pi (GST-pi) expression in renal cell carcinoma. J. Kidney Cancer VHL 2015, 2, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Rodilla, V.; Benzie, A.A.; Veitch, J.M.; Murray, G.I.; Rowe, J.D.; Hawksworth, G.M. Glutathione S-transferases in human renal cortex and neoplastic tissue: Enzymatic activity, isoenzyme profile and immunohistochemical localization. Xenobiotica Fate Foreign Compd. Biol. Syst. 1998, 28, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Kolwijck, E.; Zusterzeel, P.L.M.; Roelofs, H.M.J.; Hendriks, J.C.; Peters, W.H.M.; Massuger, L.F.A.G. GSTP1-1 in ovarian cyst fluid and disease outcome of patients with ovarian cancer. Cancer Epidemiol. Prev. Biomark. 2009, 18, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Soh, Y.; Goto, S.; Kitajima, M.; Moriyama, S.; Kotera, K.; Nakayama, T.; Nakajima, H.; Kondo, T.; Ishimaru, T. Nuclear localisation of glutathione S-transferase pi is an evaluation factor for drug resistance in gynaecological cancers. Clin. Oncol. 2005, 17, 264–270. [Google Scholar] [CrossRef]
- Oguztuzun, S.; Abu-Hijleh, A.; Coban, T.; Bulbul, D.; Kilic, M.; Iscan, M.; Iscan, M. GST isoenzymes in matched normal and neoplastic breast tissue. Neoplasma 2011, 58, 304–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreenath, A.S.; Kumar, K.R.; Reddy, G.V.; Sreedevi, B.; Praveen, D.; Monika, S.; Sudha, S.; Reddy, M.G.; Reddanna, P. Evidence for the association of synaptotagmin with glutathione S-transferases: Implications for a novel function in human breast cancer. Clin. Biochem. 2005, 38, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Sethi, S.K. Biomarkers in cardiorenal syndromes. Transl. Res. J. Lab. Clin. Med. 2014, 164, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Beyerle, J.; Frei, E.; Stiborova, M.; Habermann, N.; Ulrich, C.M. Biotransformation of xenobiotics in the human colon and rectum and its association with colorectal cancer. Drug Metab. Rev. 2015, 47, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Djukic, T.; Simic, T.; Pljesa-Ercegovac, M.; Matic, M.; Suvakov, S.; Coric, V.; Dragicevic, D.; Savic-Radojevic, A. Upregulated glutathione transferase omega-1 correlates with progression of urinary bladder carcinoma. Redox Rep. Commun. Free Radic. Res. 2017, 22, 486–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, Q.; Peng, B.; Shao, Q.; Qian, W.; Zhang, J.-Y. Identification of glutathione S-transferase omega 1 (GSTO1) protein as a novel tumor-associated antigen and its autoantibody in human esophageal squamous cell carcinoma. Tumor Biol. 2014, 35, 10871–10877. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Ni, R.-Z.; Xiao, M.-B.; Guo, J.-G.; Zhou, J.-W. Comparative proteomic analysis of differentially expressed proteins in human pancreatic cancer tissue. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 193–200. [Google Scholar] [PubMed]
- Lu, H.; Chen, I.; Shimoda, L.A.; Park, Y.; Zhang, C.; Tran, L.; Zhang, H.; Semenza, G.L. Chemotherapy-Induced Ca 2+ Release Stimulates Breast Cancer Stem Cell Enrichment. Cell Rep. 2017, 18, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Pierl, C.; Harth, V.; Pesch, B.; Rabstein, S.; Brüning, T.; Ko, Y.; Hamann, U.; Justenhoven, C.; Brauch, H.; et al. Expression of xenobiotic and steroid hormone metabolizing enzymes in human breast carcinomas. Int. J. Cancer 2006, 119, 1785–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuo, R.; Kosak, K.M.; Sankar, S.; Wiles, E.T.; Sun, Y.; Zhang, J.; Ayello, J.; Prestwich, G.D.; Shami, P.J.; Cairo, M.S.; et al. Targeting Glutathione S-transferase M4 in Ewing sarcoma. Front. Pediatr. 2014, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasello, M.; Michelacci, F.; Scionti, I.; Hattinger, C.M.; Zuntini, M.; Caccuri, A.M.; Scotlandi, K.; Picci, P.; Serra, M. Overcoming Glutathione S-Transferase P1-Related Cisplatin Resistance in Osteosarcoma. Cancer Res. 2008, 68, 6661–6668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, J.A.; Murray, G.I.; Ewen, S.W.B.; Melvin, W.T.; Burke, M.D. Immunohistochemical localization of glutathione s-transferases in sarcomas. J. Pathol. 1994, 174, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Pan, L.; Yuan, Y.; Lang, J.; Mao, N. Identification of Platinum-Resistance Associated Proteins through Proteomic Analysis of Human Ovarian Cancer Cells and Their Platinum-Resistant Sublines. J. Proteome Res. 2007, 6, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Piaggi, S.; Raggi, C.; Corti, A.; Pitzalis, E.; Mascherpa, M.C.; Saviozzi, M.; Pompella, A.; Casini, A.F. Glutathione transferase omega 1-1 (GSTO1-1) plays an anti-apoptotic role in cell resistance to cisplatin toxicity. Carcinogenesis 2010, 31, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Sinning, I.; Kleywegt, G.J.; Cowan, S.W.; Reinemer, P.; Dirr, H.W.; Huber, R.; Gilliland, G.L.; Armstrong, R.N.; Ji, X.; Board, P.G. Structure determination and refinement of human alpha class glutathione transferase A1-1, and a comparison with the Mu and Pi class enzymes. J. Mol. Biol. 1993, 232, 192–212. [Google Scholar] [CrossRef] [PubMed]
- Polekhina, G.; Board, P.G.; Blackburn, A.C.; Parker, M.W. Crystal structure of maleylacetoacetate isomerase/glutathione transferase zeta reveals the molecular basis for its remarkable catalytic promiscuity. Biochemistry 2001, 40, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Rossjohn, J.; McKinstry, W.J.; Oakley, A.J.; Verger, D.; Flanagan, J.; Chelvanayagam, G.; Tan, K.L.; Board, P.G.; Parker, M.W. Human theta class glutathione transferase: The crystal structure reveals a sulfate-binding pocket within a buried active site. Struct. Lond. Engl. 1998, 6, 309–322. [Google Scholar] [CrossRef]
- Board, P.G.; Coggan, M.; Chelvanayagam, G.; Easteal, S.; Jermiin, L.S.; Schulte, G.K.; Danley, D.E.; Hoth, L.R.; Griffor, M.C.; Kamath, A.V.; et al. Identification, characterization, and crystal structure of the Omega class glutathione transferases. J. Biol. Chem. 2000, 275, 24798–24806. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem. Sci. 1992, 17, 463–468. [Google Scholar] [CrossRef]
- Dourado, D.; Fernandes, P.; Ramos, M. Mammalian Cytosolic Glutathione Transferases. Curr. Protein Pept. Sci. 2008, 9, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cañón, J.M.; Hejna, J.; Reifsteck, C.; Olson, S.; Grompe, M. Gene structure, chromosomal location, and expression pattern of maleylacetoacetate isomerase. Genomics 1999, 58, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Beuckmann, C.T.; Fujimori, K.; Urade, Y.; Hayaishi, O. Identification of mu-class glutathione transferases M2-2 and M3-3 as cytosolic prostaglandin E synthases in the human brain. Neurochem. Res. 2000, 25, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Bogaards, J.J.; Venekamp, J.C.; van Bladeren, P.J. Stereoselective conjugation of prostaglandin A2 and prostaglandin J2 with glutathione, catalyzed by the human glutathione S-transferases A1-1, A2-2, M1a-1a, and P1-1. Chem. Res. Toxicol. 1997, 10, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Manevich, Y.; He, L.; Hutchens, S.; Pazoles, C.J.; Tew, K.D. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J. Biol. Chem. 2009, 284, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Whitbread, A.K.; Masoumi, A.; Tetlow, N.; Schmuck, E.; Coggan, M.; Board, P.G. Characterization of the omega class of glutathione transferases. Methods Enzymol. 2005, 401, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Pandya, U.; Srivastava, S.K.; Singhal, S.S.; Pal, A.; Awasthi, S.; Zimniak, P.; Awasthi, Y.C.; Singh, S.V. Activity of allelic variants of Pi class human glutathione S-transferase toward chlorambucil. Biochem. Biophys. Res. Commun. 2000, 278, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Tew, K.D. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994, 54, 4313–4320. [Google Scholar] [CrossRef] [PubMed]
- McLellan, L.I.; Wolf, C.R. Glutathione and glutathione-dependent enzymes in cancer drug resistance. Drug Resist. Updat. 1999, 2, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-W.; Ali-Osman, F. Genetic polymorphism and function of glutathione S-transferases in tumor drug resistance. Curr. Opin. Pharmacol. 2007, 7, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Roco, A.; Cayún, J.; Contreras, S.; Stojanova, J.; Quiñones, L. Can pharmacogenetics explain efficacy and safety of cisplatin pharmacotherapy? Front. Genet. 2014, 5, 391. [Google Scholar] [CrossRef] [PubMed]
- Huezo-Diaz, P.; Uppugunduri, C.R.S.; Tyagi, A.K.; Krajinovic, M.; Ansari, M. Pharmacogenetic aspects of drug metabolizing enzymes in busulfan based conditioning prior to allogenic hematopoietic stem cell transplantation in children. Curr. Drug Metab. 2014, 15, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Ekhart, C.; Doodeman, V.D.; Rodenhuis, S.; Smits, P.H.M.; Beijnen, J.H.; Huitema, A.D.R. Polymorphisms of drug-metabolizing enzymes (GST, CYP2B6 and CYP3A) affect the pharmacokinetics of thiotepa and tepa. Br. J. Clin. Pharmacol. 2009, 67, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertholee, D.; Maring, J.G.; van Kuilenburg, A.B.P. Genotypes Affecting the Pharmacokinetics of Anticancer Drugs. Clin. Pharmacokinet. 2017, 56, 317–337. [Google Scholar] [CrossRef] [PubMed]
- Gaziev, J.; Nguyen, L.; Puozzo, C.; Mozzi, A.F.; Casella, M.; Perrone Donnorso, M.; Gravina, P.; Sodani, P.; Marziali, M.; Isgro, A.; et al. Novel pharmacokinetic behavior of intravenous busulfan in children with thalassemia undergoing hematopoietic stem cell transplantation: A prospective evaluation of pharmacokinetic and pharmacodynamic profile with therapeutic drug monitoring. Blood 2010, 115, 4597–4604. [Google Scholar] [CrossRef] [PubMed]
- Ekhart, C.; Rodenhuis, S.; Smits, P.H.M.; Beijnen, J.H.; Huitema, A.D.R. Relations between polymorphisms in drug-metabolising enzymes and toxicity of chemotherapy with cyclophosphamide, thiotepa and carboplatin. Pharmacogenet. Genom. 2008, 18, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Tew, K.D.; Monks, A.; Barone, L.; Rosser, D.; Akerman, G.; Montali, J.A.; Wheatley, J.B.; Schmidt, D.E. Glutathione-associated enzymes in the human cell lines of the National Cancer Institute Drug Screening Program. Mol. Pharmacol. 1996, 50, 149–159. [Google Scholar] [PubMed]
- Townsend, D.M.; Tew, K.D. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 2003, 22, 7369–7375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, F.; Nakanishi, Y.; Kawasaki, M.; Takayama, K.; Yatsunami, J.; Pei, X.H.; Tsuruta, N.; Wakamatsu, K.; Hara, N. Immunohistochemical expression of glutathione S-transferase-Pi can predict chemotherapy response in patients with nonsmall cell lung carcinoma. Cancer 1996, 78, 416–421. [Google Scholar] [CrossRef]
- Black, S.M.; Beggs, J.D.; Hayes, J.D.; Bartoszek, A.; Muramatsu, M.; Sakai, M.; Wolf, C.R. Expression of human glutathione S-transferases in Saccharomyces cerevisiae confers resistance to the anticancer drugs adriamycin and chlorambucil. Biochem. J. 1990, 268, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Ban, N.; Takahashi, Y.; Takayama, T.; Kura, T.; Katahira, T.; Sakamaki, S.; Niitsu, Y. Transfection of glutathione S-transferase (GST)-pi antisense complementary DNA increases the sensitivity of a colon cancer cell line to adriamycin, cisplatin, melphalan, and etoposide. Cancer Res. 1996, 56, 3577–3582. [Google Scholar] [PubMed]
- Lewis, A.D.; Hickson, I.D.; Robson, C.N.; Harris, A.L.; Hayes, J.D.; Griffiths, S.A.; Manson, M.M.; Hall, A.E.; Moss, J.E.; Wolf, C.R. Amplification and increased expression of alpha class glutathione S-transferase-encoding genes associated with resistance to nitrogen mustards. Proc. Natl. Acad. Sci. USA 1988, 85, 8511–8515. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.M.; Williamson, C.; Hall, A.G.; Elgie, A.W.; Taylor, C.G. Evidence for the involvement of the glutathione pathway in drug resistance in AML. Adv. Exp. Med. Biol. 1999, 457, 205–209. [Google Scholar] [PubMed]
- Sharma, A.; Patrick, B.; Li, J.; Sharma, R.; Jeyabal, P.V.S.; Reddy, P.M.R.V.; Awasthi, S.; Awasthi, Y.C. Glutathione S-transferases as antioxidant enzymes: Small cell lung cancer (H69) cells transfected with hGSTA1 resist doxorubicin-induced apoptosis. Arch. Biochem. Biophys. 2006, 452, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Finn, N.A.; Kemp, M.L. Pro-oxidant and antioxidant effects of N-acetylcysteine regulate doxorubicin-induced NF-kappa B activity in leukemic cells. Mol. Biosyst. 2012, 8, 650–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Board, P.G.; Menon, D. Structure, function and disease relevance of Omega-class glutathione transferases. Arch. Toxicol. 2016, 90, 1049–1067. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Gallagher, E. From JNK to pay dirt: Jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life 2005, 57, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Adler, V.; Yin, Z.; Fuchs, S.Y.; Benezra, M.; Rosario, L.; Tew, K.D.; Pincus, M.R.; Sardana, M.; Henderson, C.J.; Wolf, C.R.; et al. Regulation of JNK signaling by GSTp. EMBO J. 1999, 18, 1321–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Fan, Y.; Xue, B.; Luo, L.; Shen, J.; Zhang, S.; Jiang, Y.; Yin, Z. Human glutathione S-transferase P1-1 interacts with TRAF2 and regulates TRAF2-ASK1 signals. Oncogene 2006, 25, 5787–5800. [Google Scholar] [CrossRef] [PubMed]
- Tew, K.D.; Manevich, Y.; Grek, C.; Xiong, Y.; Uys, J.; Townsend, D.M. The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free Radic. Biol. Med. 2011, 51, 299–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tew, K.D. Redox in redux: Emergent roles for glutathione S-transferase P (GSTP) in regulation of cell signaling and S-glutathionylation. Biochem. Pharmacol. 2007, 73, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Thévenin, A.F.; Zony, C.L.; Bahnson, B.J.; Colman, R.F. GST pi modulates JNK activity through a direct interaction with JNK substrate, ATF2. Protein Sci. Publ. Protein Soc. 2011, 20, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.; Andrews, K.; Ng, L.; O’Rourke, K.; Maslen, A.; Kirby, G. Human GSTA1-1 reduces c-Jun N-terminal kinase signalling and apoptosis in Caco-2 cells. Biochem. J. 2006, 400, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.G.; Lee, Y.H.; Park, H.S.; Ryoo, K.; Kang, K.W.; Park, J.; Eom, S.J.; Kim, M.J.; Chang, T.S.; Choi, S.Y.; et al. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J. Biol. Chem. 2001, 276, 12749–12755. [Google Scholar] [CrossRef] [PubMed]
- Ichijo, H.; Nishida, E.; Irie, K.; ten Dijke, P.; Saitoh, M.; Moriguchi, T.; Takagi, M.; Matsumoto, K.; Miyazono, K.; Gotoh, Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997, 275, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Dorion, S.; Lambert, H.; Landry, J. Activation of the p38 signaling pathway by heat shock involves the dissociation of glutathione S-transferase Mu from Ask1. J. Biol. Chem. 2002, 277, 30792–30797. [Google Scholar] [CrossRef] [PubMed]
- Coric, V.M.; Simic, T.P.; Pekmezovic, T.D.; Basta-Jovanovic, G.M.; Savic-Radojevic, A.R.; Radojevic-Skodric, S.M.; Matic, M.G.; Suvakov, S.R.; Dragicevic, D.P.; Radic, T.M.; et al. GSTM1 genotype is an independent prognostic factor in clear cell renal cell carcinoma. Urol. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Kruh, G.D.; Tew, K.D. The influence of coordinate overexpression of glutathione phase II detoxification gene products on drug resistance. J. Pharmacol. Exp. Ther. 2000, 294, 480–487. [Google Scholar] [PubMed]
- Smitherman, P.K.; Townsend, A.J.; Kute, T.E.; Morrow, C.S. Role of multidrug resistance protein 2 (MRP2, ABCC2) in alkylating agent detoxification: MRP2 potentiates glutathione S-transferase A1-1-mediated resistance to chlorambucil cytotoxicity. J. Pharmacol. Exp. Ther. 2004, 308, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Depeille, P.; Cuq, P.; Mary, S.; Passagne, I.; Evrard, A.; Cupissol, D.; Vian, L. Glutathione S-transferase M1 and multidrug resistance protein 1 act in synergy to protect melanoma cells from vincristine effects. Mol. Pharmacol. 2004, 65, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Seitz, G.; Bonin, M.; Fuchs, J.; Poths, S.; Ruck, P.; Warmann, S.W.; Armeanu-Ebinger, S. Inhibition of glutathione-S-transferase as a treatment strategy for multidrug resistance in childhood rhabdomyosarcoma. Int. J. Oncol. 2010, 36, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Batist, G. Glutathione and glutathione analogues; therapeutic potentials. Biochim. Biophys. Acta 2013, 1830, 3350–3353. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione transferases: Substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; Lee, K.-H.; Chang, F.-R.; Chuang, D.-W.; Yang, J.-C. Compound for Inhibiting Activity of Glutathione S-Transferase Omega 1 and Preparation Method Thereof, and Pharmaceutical Compositions Containing Compound. Patent CN2013/088871, 18 June 2015. [Google Scholar]
- Awasthi, S.; Srivastava, S.K.; Ahmad, F.; Ahmad, H.; Ansari, G.A. Interactions of glutathione S-transferase-pi with ethacrynic acid and its glutathione conjugate. Biochim. Biophys. Acta 1993, 1164, 173–178. [Google Scholar] [CrossRef]
- Ploemen, J.H.; van Ommen, B.; Bogaards, J.J.; van Bladeren, P.J. Ethacrynic acid and its glutathione conjugate as inhibitors of glutathione S-transferases. Xenobiotica Fate Foreign Compd. Biol. Syst. 1993, 23, 913–923. [Google Scholar] [CrossRef]
- Zhang, J.; Nishimoto, Y.; Tokuda, H.; Suzuki, N.; Yasukawa, K.; Kitdamrongtham, W.; Akazawa, H.; Manosroi, A.; Manosroi, J.; Akihisa, T. Cancer chemopreventive effect of bergenin from Peltophorum pterocarpum wood. Chem. Biodivers. 2013, 10, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; El Brahmi, N.; El Kazzouli, S.; Eloy, L.; Courilleau, D.; Caron, J.; Bousmina, M.M.; Caminade, A.-M.; Cresteil, T.; Majoral, J.-P. A novel class of ethacrynic acid derivatives as promising drug-like potent generation of anticancer agents with established mechanism of action. Eur. J. Med. Chem. 2016, 122, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.; Dutta, S.; Tew, K.D. Inhibitors of glutathione S-transferases as therapeutic agents. Adv. Drug Deliv. Rev. 1997, 26, 91–104. [Google Scholar] [CrossRef]
- Parker, L.J.; Italiano, L.C.; Morton, C.J.; Hancock, N.C.; Ascher, D.B.; Aitken, J.B.; Harris, H.H.; Campomanes, P.; Rothlisberger, U.; De Luca, A.; et al. Studies of glutathione transferase P1-1 bound to a platinum(IV)-based anticancer compound reveal the molecular basis of its activation. Chem. Weinh. Bergstr. Ger. 2011, 17, 7806–7816. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, C.; Jin, S.; Liu, J.; Xue, X.; Eltahan, A.S.; Sun, J.; Tan, J.; Dong, J.; Liang, X.-J. Overcoming resistance to cisplatin by inhibition of glutathione S-transferases (GSTs) with ethacraplatin micelles in vitro and in vivo. Biomaterials 2017, 144, 119–129. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Hartinger, C.G.; Dyson, P.J.; Lo Bello, M.; Casini, A. A new target for gold(I) compounds: Glutathione-S-transferase inhibition by auranofin. J. Inorg. Biochem. 2013, 119, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, I.; Caccuri, A.M.; Cocco, A.; De Maria, F.; Di Stefano, A.; Luisi, G.; Pinnen, F.; Ricci, G.; Sozio, P.; Turella, P. Potent isozyme-selective inhibition of human glutathione S-transferase A1-1 by a novel glutathione S-conjugate. Amino Acids 2005, 29, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Burg, D.; Riepsaame, J.; Pont, C.; Mulder, G.; van de Water, B. Peptide-bond modified glutathione conjugate analogs modulate GSTpi function in GSH-conjugation, drug sensitivity and JNK signaling. Biochem. Pharmacol. 2006, 71, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Galili, N.; Smith, S.; Godwin, J.; Lancet, J.; Melchert, M.; Jones, M.; Keck, J.G.; Meng, L.; Brown, G.L.; et al. Phase 1 multicenter dose-escalation study of ezatiostat hydrochloride (TLK199 tablets), a novel glutathione analog prodrug, in patients with myelodysplastic syndrome. Blood 2009, 113, 6533–6540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruscoe, J.E.; Rosario, L.A.; Wang, T.; Gaté, L.; Arifoglu, P.; Wolf, C.R.; Henderson, C.J.; Ronai, Z.; Tew, K.D. Pharmacologic or genetic manipulation of glutathione S-transferase P1-1 (GSTpi) influences cell proliferation pathways. J. Pharmacol. Exp. Ther. 2001, 298, 339–345. [Google Scholar] [PubMed]
- Hamilton, D.; Batist, G. TLK-199 (Telik). IDrugs Investig. Drugs J. 2005, 8, 662–669. [Google Scholar]
- Tentori, L.; Dorio, A.S.; Mazzon, E.; Muzi, A.; Sau, A.; Cuzzocrea, S.; Vernole, P.; Federici, G.; Caccuri, A.M.; Graziani, G. The glutathione transferase inhibitor 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX) increases temozolomide efficacy against malignant melanoma. Eur. J. Cancer 2011, 47, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Federici, L.; Lo Sterzo, C.; Pezzola, S.; Di Matteo, A.; Scaloni, F.; Federici, G.; Caccuri, A.M. Structural basis for the binding of the anticancer compound 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol to human glutathione s-transferases. Cancer Res. 2009, 69, 8025–8034. [Google Scholar] [CrossRef] [PubMed]
- Fulci, C.; Rotili, D.; De Luca, A.; Stella, L.; Morozzo Della Rocca, B.; Forgione, M.; Di Paolo, V.; Mai, A.; Falconi, M.; Quintieri, L.; et al. A new nitrobenzoxadiazole-based GSTP1-1 inhibitor with a previously unheard of mechanism of action and high stability. J. Enzyme Inhib. Med. Chem. 2017, 32, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sha, H.-H.; Wang, Z.; Dong, S.-C.; Hu, T.-M.; Liu, S.-W.; Zhang, J.-Y.; Wu, Y.; Ma, R.; Wu, J.-Z.; Chen, D.; et al. 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio) hexanol: A promising new anticancer compound. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Dahlin, J.L.; Oakley, A.J.; Casarotto, M.G.; Board, P.G.; Baell, J.B. Reviewing Hit Discovery Literature for Difficult Targets: Glutathione Transferase Omega-1 as an Example. J. Med. Chem. 2018, 61, 7448–7470. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Bachovchin, D.A.; Speers, A.E.; Brown, S.J.; Spicer, T.; Fernandez-Vega, V.; Ferguson, J.; Cravatt, B.F.; Hodder, P.; Rosen, H. Optimization and Characterization of an Inhibitor for Glutathione S-Tranferase Omega 1 (GSTO1). In Probe Reports from the NIH Molecular Libraries Program; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010. [Google Scholar]
- Tsuboi, K.; Bachovchin, D.A.; Speers, A.E.; Spicer, T.P.; Fernandez-Vega, V.; Hodder, P.; Rosen, H.; Cravatt, B.F. Potent and Selective Inhibitors of Glutathione S-Transferase Omega 1 That Impair Cancer Drug Resistance. J. Am. Chem. Soc. 2011, 133, 16605–16616. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.; Coll, R.; O’Neill, L.A.J.; Board, P.G. Glutathione transferase Omega 1 is required for the lipopolysaccharide-stimulated induction of NADPH oxidase 1 and the production of reactive oxygen species in macrophages. Free Radic. Biol. Med. 2014, 73, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, K.; Samanta, S.; Kyani, A.; Yang, S.; Tamura, S.; Ziemke, E.; Stuckey, J.A.; Li, S.; Chinnaswamy, K.; Otake, H.; et al. Mechanistic evaluation and transcriptional signature of a glutathione S-transferase omega 1 inhibitor. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Pace, N.J.; Pimental, D.R.; Weerapana, E. An Inhibitor of Glutathione S-Transferase Omega 1 that Selectively Targets Apoptotic Cells. Angew. Chem. Int. Ed. 2012, 51, 8365–8368. [Google Scholar] [CrossRef] [PubMed]
- Kilpin, K.J.; Dyson, P.J. Enzyme inhibition by metal complexes: Concepts, strategies and applications. Chem. Sci. 2013, 4, 1410. [Google Scholar] [CrossRef]
- Porchia, M.; Pellei, M.; Marinelli, M.; Tisato, F.; Del Bello, F.; Santini, C. New insights in Au-NHCs complexes as anticancer agents. Eur. J. Med. Chem. 2018, 146, 709–746. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, E.G.; Labrou, N.E. Glutathione transferases: Emerging multidisciplinary tools in red and green biotechnology. Recent Pat. Biotechnol. 2009, 3, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Geroni, C.; Marchini, S.; Cozzi, P.; Galliera, E.; Ragg, E.; Colombo, T.; Battaglia, R.; Howard, M.; D’Incalci, M.; Broggini, M. Brostallicin, a novel anticancer agent whose activity is enhanced upon binding to glutathione. Cancer Res. 2002, 62, 2332–2336. [Google Scholar] [PubMed]
- Van Haaften, R.I.M.; Haenen, G.R.M.M.; van Bladeren, P.J.; Bogaards, J.J.P.; Evelo, C.T.A.; Bast, A. Inhibition of various glutathione S-transferase isoenzymes by RRR-alpha-tocopherol. Toxicol. Vitro 2003, 17, 245–251. [Google Scholar] [CrossRef]
- Sampayo-Reyes, A.; Zakharyan, R.A. Tocopherol esters inhibit human glutathione S-transferase omega. Acta Biochim. Pol. 2006, 53, 547–552. [Google Scholar] [PubMed]
- Angulo-Molina, A.; Reyes-Leyva, J.; López-Malo, A.; Hernández, J. The role of alpha tocopheryl succinate (α-TOS) as a potential anticancer agent. Nutr. Cancer 2014, 66, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Sachs, G. Pharmacology of proton pump inhibitors. Curr. Gastroenterol. Rep. 2008, 10, 528–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural Mechanism for Rifampicin Inhibition of Bacterial RNA Polymerase. Cell 2001, 104, 901–912. [Google Scholar] [CrossRef]
- Bachovchin, D.A.; Brown, S.J.; Rosen, H.; Cravatt, B.F. Identification of selective inhibitors of uncharacterized enzymes by high-throughput screening with fluorescent activity-based probes. Nat. Biotechnol. 2009, 27, 387–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Milito, A.; Fais, S. Proton pump inhibitors may reduce tumour resistance. Expert Opin. Pharmacother. 2005, 6, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Kojima, F.; Yamazaki, T.; Tatee, T.; Abe, F.; Muraoka, Y.; Naganawa, H.; Aoyagi, T.; Takeuchi, T. Benastatins C and D, new inhibitors of glutathione S-transferase, produced by Streptomyces sp. MI384-DF12. Production, isolation, structure determination and biological activities. J. Antibiot. 1993, 46, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Shen, J.; Lu, D.; Zhang, T.; Zhang, W.; Sun, D.; Wang, P.G. 4-Aryl-1,3,2-oxathiazolylium-5-olate: A novel GST inhibitor to release JNK and activate c-Jun for cancer therapy. Cancer Chemother. Pharmacol. 2008, 62, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Harshbarger, W.; Gondi, S.; Ficarro, S.B.; Hunter, J.; Udayakumar, D.; Gurbani, D.; Singer, W.D.; Liu, Y.; Li, L.; Marto, J.A.; et al. Structural and Biochemical Analyses Reveal the Mechanism of Glutathione S-Transferase Pi 1 Inhibition by the Anti-cancer Compound Piperlongumine. J. Biol. Chem. 2017, 292, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.-H.S.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Tome, M.E.; Ranger-Moore, J.; Cordova, C.A.; Mikhael, D.M.; Briehl, M.M.; Alberts, D.S. Modulation of human glutathione s-transferases by polyphenon e intervention. Cancer Epidemiol. Prev. Biomark. 2007, 16, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-W.; Liu, K.-L.; Lin, C.-Y.; Chen, H.-W.; Lii, C.-K. Structure and function relationship study of allium organosulfur compounds on upregulating the pi class of glutathione S-transferase expression. J. Agric. Food Chem. 2011, 59, 3398–3405. [Google Scholar] [CrossRef] [PubMed]
- Schulz, W.A.; Hatina, J. Epigenetics of prostate cancer: Beyond DNA methylation. J. Cell. Mol. Med. 2006, 10, 100–125. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Barve, A.; Khor, T.-O.; Shen, G.; Lin, W.; Chan, J.Y.; Cai, L.; Kong, A.-N. Regulation of Nrf2- and AP-1-mediated gene expression by epigallocatechin-3-gallate and sulforaphane in prostate of Nrf2-knockout or C57BL/6J mice and PC-3 AP-1 human prostate cancer cells. Acta Pharmacol. Sin. 2010, 31, 1223–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauptstock, V.; Kuriakose, S.; Schmidt, D.; Düster, R.; Müller, S.C.; von Ruecker, A.; Ellinger, J. Glutathione-S-transferase pi 1(GSTP1) gene silencing in prostate cancer cells is reversed by the histone deacetylase inhibitor depsipeptide. Biochem. Biophys. Res. Commun. 2011, 412, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Ruzza, P.; Calderan, A. Glutathione Transferase (GST)-Activated Prodrugs. Pharmaceutics 2013, 5, 220–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.-C.; Sha, H.-H.; Xu, X.-Y.; Hu, T.-M.; Lou, R.; Li, H.; Wu, J.-Z.; Dan, C.; Feng, J. Glutathione S-transferase π: A potential role in antitumor therapy. Drug Des. Dev. Ther. 2018, 12, 3535–3547. [Google Scholar] [CrossRef]
- Ramsay, E.E.; Dilda, P.J. Glutathione S-conjugates as prodrugs to target drug-resistant tumors. Front. Pharmacol. 2014, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Findlay, V.J.; Townsend, D.M.; Saavedra, J.E.; Buzard, G.S.; Citro, M.L.; Keefer, L.K.; Ji, X.; Tew, K.D. Tumor cell responses to a novel glutathione S-transferase-activated nitric oxide-releasing prodrug. Mol. Pharmacol. 2004, 65, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Pal, A.; Kalathur, R.; Hu, X.; Gu, Y.; Saavedra, J.E.; Buzard, G.S.; Srinivasan, A.; Keefer, L.K.; Singh, S.V. Structure-Based Design of Anticancer Prodrug PABA/NO. Drug Des. Dev. Ther. 2008, 2, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Burke, P.J.; Wong, L.C.; Jenkins, T.C.; Knox, R.J.; Meikle, I.T.; Stanforth, S.P. Studies relating to the synthesis, enzymatic reduction and cytotoxicity of a series of nitroaromatic prodrugs. Bioorg. Med. Chem. Lett. 2016, 26, 5851–5854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, R.; Wu, J.; Luo, X.; Gong, Y.; Huang, Y.; Shen, X.; Zhang, H.; Zhang, Y.; Huang, Z. Design, Synthesis, and Evaluation of Diazeniumdiolate-Based DNA Cross-Linking Agents Activatable by Glutathione S-Transferase. Org. Lett. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kiziltepe, T.; Anderson, K.C.; Kutok, J.L.; Jia, L.; Boucher, K.M.; Saavedra, J.E.; Keefer, L.K.; Shami, P.J. JS-K has potent anti-angiogenic activity in vitro and inhibits tumour angiogenesis in a multiple myeloma model in vivo. J. Pharm. Pharmacol. 2010, 62, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Tew, K.D. TLK-286: A novel glutathione S-transferase-activated prodrug. Expert Opin. Investig. Drugs 2005, 14, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Finkler, N.J.; Hall, J.B.; Melnyk, O.; Edwards, R.P.; Jones, M.; Keck, J.G.; Meng, L.; Brown, G.L.; Rankin, E.M.; et al. Randomized phase III study of canfosfamide in combination with pegylated liposomal doxorubicin compared with pegylated liposomal doxorubicin alone in platinum-resistant ovarian cancer. Int. J. Gynecol. Cancer 2010, 20, 772–780. [Google Scholar] [CrossRef]
- Dourado, D.F.A.R.; Fernandes, P.A.; Ramos, M.J.; Mannervik, B. Mechanism of glutathione transferase P1-1-catalyzed activation of the prodrug canfosfamide (TLK286, TELCYTA). Biochemistry 2013, 52, 8069–8078. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.S.; Laxa, B.; Boulos, L.; Wiggins, L.; Keck, J.G.; Jameson, A.J.; Parra, R.; Patel, K.; Brown, G.L. Phase 1 study of TLK286 (Telcyta) administered weekly in advanced malignancies. Clin. Cancer Res. 2004, 10, 3689–3698. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, J.J.; Gershenson, D.M.; Choi, H.; Lewis, L.; Patel, K.; Brown, G.L.; Garcia, A.; Spriggs, D.R. Multi-institutional phase 2 study of TLK286 (TELCYTA, a glutathione S-transferase P1-1 activated glutathione analog prodrug) in patients with platinum and paclitaxel refractory or resistant ovarian cancer. Int. J. Gynecol. Cancer 2005, 15, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Mainenti, S.; Pietragalla, A.; Ferrandina, G.; Foco, G.; Masciullo, V.; Scambia, G. Brostallicin (PNU-166196), a new minor groove DNA binder: Preclinical and clinical activity. Expert Opin. Investig. Drugs 2009, 18, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Pezzola, S.; Antonini, G.; Geroni, C.; Beria, I.; Colombo, M.; Broggini, M.; Marchini, S.; Mongelli, N.; Leboffe, L.; MacArthur, R.; et al. Role of glutathione transferases in the mechanism of brostallicin activation. Biochemistry 2010, 49, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattinger, C.M.; Pasello, M.; Ferrari, S.; Picci, P.; Serra, M. Emerging drugs for high-grade osteosarcoma. Expert Opin. Emerg. Drugs 2010, 15, 615–634. [Google Scholar] [CrossRef] [PubMed]
- Axarli, I.; Labrou, N.E.; Petrou, C.; Rassias, N.; Cordopatis, P.; Clonis, Y.D. Sulphonamide-based bombesin prodrug analogues for glutathione transferase, useful in targeted cancer chemotherapy. Eur. J. Med. Chem. 2009, 44, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Ikhlas, S.; Ahmad, M. Metformin: Insights into its anticancer potential with special reference to AMPK dependent and independent pathways. Life Sci. 2017, 185, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Vernerová, M.; Aufderhaar, I.; Huttunen, K.M. Glutathione-S-transferase selective release of metformin from its sulfonamide prodrug. Bioorg. Med. Chem. Lett. 2014, 24, 5034–5036. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, K.M.; Rautio, J.; Leppänen, J.; Vepsäläinen, J.; Keski-Rahkonen, P. Determination of metformin and its prodrugs in human and rat blood by hydrophilic interaction liquid chromatography. J. Pharm. Biomed. Anal. 2009, 50, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Van Gisbergen, M.W.; Cebula, M.; Zhang, J.; Ottosson-Wadlund, A.; Dubois, L.; Lambin, P.; Tew, K.D.; Townsend, D.M.; Haenen, G.R.M.M.; Drittij-Reijnders, M.-J.; et al. Chemical Reactivity Window Determines Prodrug Efficiency toward Glutathione Transferase Overexpressing Cancer Cells. Mol. Pharm. 2016, 13, 2010–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlin, I. The Hedgehog and the Fox: An Essay on Tolstoy’s View of History, 1st Elephant paperback ed.; Ivan R. Dee, Publisher: Chicago, IL, USA, 1993; ISBN 978-1-56663-019-1. [Google Scholar]


| Anti-Cancer Drug | GST Class | GST Polymorphism Influencing Drug Response |
|---|---|---|
| Detoxification by means of glutathione conjugation | ||
| BCNU/Carmustine | Alpha | Unknown [64,68] |
| Mu | GSTM1, GSTM3–Unknown [29,68] | |
| Theta | GSTT1–Unknown [29,68] | |
| Busulfran | Alpha, predominantly | GSTA1*B (-567G, -69T, -52A) [66,69] |
| Brostallicin | Alpha, Mu, Pi | Unknown [64,68] |
| Carboplatin | Pi, Alpha | Unknown [70] |
| Chlorambucil | Alpha | GSTA1*A (-567T, -69C, -52G) [1] |
| GSTA2-2, point mutations in exon 5 and 7 [29,68] | ||
| Pi | GSTP1*A (Ile105/Ala114) [61] | |
| Cisplatin, oxaliplatin | Pi, Mu, Theta | Controversial [65] |
| Cyclophosphamide | Alpha | GSTA1*B (-567G, -69T, -52A) [29,68] |
| Pi | GSTP1*B (Val105/Ala114) [29,68] | |
| Etoposide | Pi | GSTP1*D (Ile105/Val114) [29,68] |
| Melphalan | Alpha | Unknown [63,64,68] |
| Pi | GSTP1*D (Ile105/Val114) [29,68] | |
| Paclitaxel, docetaxel | Pi | GSTP1*C (Val105/Val114) [29,68] |
| Thiotepa | Alpha | GSTA1*A (-567T, -69C, -52G) [29,68] |
| Mu | Point mutation in exone 7 [29,68] | |
| Pi | GSTP1*A (Ile105/Ala114) | |
| GSTP1 (Ala114Val) [67] | ||
| Thiopurines | Alpha, Mu | Unknown [64,68] |
| Detoxification by means of redox regulation | ||
| Doxorubicin | Pi | GSTP1, point mutations in exons 5 and 6 [29] |
| Other anthracyclines | Alpha | GSTA4–4, Unknown [63] |
| Other anti-cancer drugs as GST substrates* | ||
| Yet to be determined | Unknown [68] | |
| GST Inhibitors and Pro-Drugs | Mechanism | Clinical Perspective | Structure |
|---|---|---|---|
| Ethacraplatin—containing micelles | enhances the accumulation of active cisplatin in GSTP1 and GSTT1 overexpressing cancer cells by inhibiting the activity of GSTs and circumventing deactivation of cisplatin | with FDA-approved adjuvant, 1,2-distearoylsn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) |  [122] |
| TLK199 | selective inhibitor of GSTP1-1 acting on MAPK signaling pathway and inhibitor of MDR-1 | completed or phase IIa clinical trial in non- small cell lung cancer and myelodysplastic syndrome |  [72,73] |
| Auranofin | GSTP1 inhibitor which enables cells to overcome resistance to platinum-based drug | completed or recruiting phase II clinical trial in ovarian cancer, small and non-small cell lung carcinoma and lung adenocarcinoma |  [123] |
| TLK286 | bio-activation by GSTP1-1 into alkylating metabolite capable of covalently binding DNA | completed phase IIa and terminated phase III clinical trial in ovarian, breast and non-small cell lung cancer |  [124] |
| Brostallicin | activated in reactions catalyzed by GSTP and GSTM | completed phase II clinical trial in breast cancer |  [125] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pljesa-Ercegovac, M.; Savic-Radojevic, A.; Matic, M.; Coric, V.; Djukic, T.; Radic, T.; Simic, T. Glutathione Transferases: Potential Targets to Overcome Chemoresistance in Solid Tumors. Int. J. Mol. Sci. 2018, 19, 3785. https://doi.org/10.3390/ijms19123785
Pljesa-Ercegovac M, Savic-Radojevic A, Matic M, Coric V, Djukic T, Radic T, Simic T. Glutathione Transferases: Potential Targets to Overcome Chemoresistance in Solid Tumors. International Journal of Molecular Sciences. 2018; 19(12):3785. https://doi.org/10.3390/ijms19123785
Chicago/Turabian StylePljesa-Ercegovac, Marija, Ana Savic-Radojevic, Marija Matic, Vesna Coric, Tatjana Djukic, Tanja Radic, and Tatjana Simic. 2018. "Glutathione Transferases: Potential Targets to Overcome Chemoresistance in Solid Tumors" International Journal of Molecular Sciences 19, no. 12: 3785. https://doi.org/10.3390/ijms19123785
APA StylePljesa-Ercegovac, M., Savic-Radojevic, A., Matic, M., Coric, V., Djukic, T., Radic, T., & Simic, T. (2018). Glutathione Transferases: Potential Targets to Overcome Chemoresistance in Solid Tumors. International Journal of Molecular Sciences, 19(12), 3785. https://doi.org/10.3390/ijms19123785