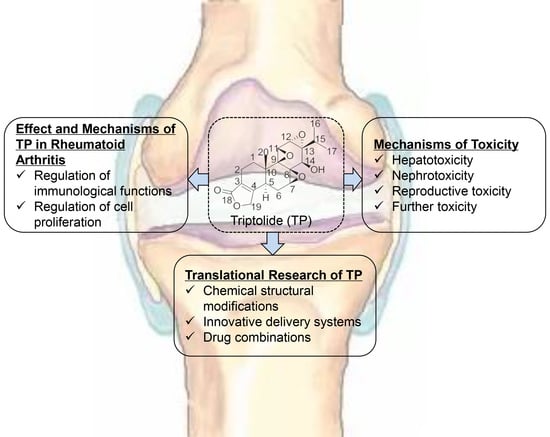
The Effect of Triptolide in Rheumatoid Arthritis: From Basic Research towards Clinical Translation
Abstract
:1. Introduction
2. Effect and Mechanisms of Triptolide (TP) in Rheumatoid Arthritis (RA)
2.1. Regulation of Immunological Functions
2.1.1. Regulation of Immune-Related Cells
2.1.2. Regulation of Immune-Related Inflammatory Mediators
2.1.3. Regulation of Immune-Related Angiogenesis
2.1.4. Regulation of Immune-Related Bone Homeostasis
2.2. Regulation of Cell Proliferation
3. Mechanisms of TP Toxicity
3.1. Hepatotoxicity
3.2. Nephrotoxicity
3.3. Reproductive Toxicity
3.4. Further Toxicity
4. Translational Research of TP
4.1. Chemical Structural Modifications of TP
4.2. Innovative Delivery System
4.2.1. Liposomes
4.2.2. Nanoparticles
4.2.3. Solid Lipid Nanoparticles
4.2.4. Microemulsions
4.3. Drug Combinations
4.3.1. Glycyrrhetinic Acid
4.3.2. Silymarin
5. Discussion and Further Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Q.; Li, G.; Sun, S.; Guo, Y.; Kuang, H. The treatment of rheumatoid arthritis using Chinese medicinal plants: From pharmacology to potential molecular mechanisms. J. Ethnopharmacol. 2015, 176, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, Y.; Zhou, X. Inhibitory effect of Triperygium wilfordii polyglucoside on dipeptidyl peptidase I in vivo and in vitro. Biomed. Pharmacother. 2017, 96, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Rostami-Yazdi, M.; Gerdes, S.; Mrowietz, U. Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br. J. Clin. Pharmacol. 2012, 74, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; He, X.; Bian, Y.; Guo, Q.; Zheng, K.; Zhao, Y.; Lu, C.; Liu, B.; Xu, X.; Zhang, G. Triptolide Modulates TREM-1 Signal Pathway to Inhibit the Inflammatory Response in Rheumatoid Arthritis. Int. J. Mol. Sci. 2016, 17, 498. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, H.; Lu, C. Triptolide Inhibits Osteoclast Differentiation and Bone Resorption In Vitro via Enhancing the Production of IL-10 and TGF-β1 by Regulatory T Cells. Mediat. Inflamm. 2016, 2016, 8048170. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Kim, K.W.; Yang, C.W.; Park, S.H.; Ju, J.H. Cytokine-mediated bone destruction in rheumatoid arthritis. J. Immunol. Res. 2014, 2014, 263625. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.A.; Kohlmeier, J.E.; Branden, M.; Jung, M.; Benedict, S.H. Triptolide is more effective in preventing T cell proliferation and interferon-gamma production than is FK506. Phytother. Res. 1999, 13, 464–467. [Google Scholar] [CrossRef]
- Mellado, M.; Martinez-Munoz, L.; Cascio, G.; Lucas, P.; Pablos, J.L.; Rodriguez-Frade, J.M. T Cell Migration in Rheumatoid Arthritis. Front. Immunol. 2015, 6, 384. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhao, L.; Liu, Z.; Lu, C.; Zhao, N.; Yang, D.; Chen, S.; Tang, J.C.; Chan, A.; Lu, A.P. The effect of triptolide on CD4+ and CD8+ cells in the Peyer’s patch of DA rats with collagen induced arthritis. Nat. Prod. Res. 2009, 23, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiao, C.; Zhao, L.; Jia, H.; Zhao, N.; Lu, C.; Yang, D.; Tang, J.C.; Chan, A.S.; Lu, A.P. The effect of triptolide on CD4+ and CD8+ cells in Peyer’s patch of SD rats with collagen induced arthritis. Int. Immunopharmacol. 2006, 6, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, A.; Zeng, H.; Liu, L.; Jiang, W.; Zhu, Y.; Xu, Y. Effect of triptolide on T-cell receptor beta variable gene mRNA expression in rats with collagen-induced arthritis. Anal. Rec. 2012, 295, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P.; Korn, T.; Kuchroo, V.K. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 2009, 361, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Volpe, E.; Servant, N.; Zollinger, R.; Bogiatzi, S.I.; Hupe, P.; Barillot, E.; Soumelis, V. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 2008, 9, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, L.; Wu, C.Y. Triptolide inhibits the differentiation of Th17 cells and suppresses collagen-induced arthritis. Scand. J. Immunol. 2008, 68, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.E.; Thomson, A.W. Dendritic cells: Regulators of alloimmunity and opportunities for tolerance induction. Immunol. Rev. 2003, 196, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Lamb, J.R.; Tam, P.K. Triptolide, a component of Chinese herbal medicine, modulates the functional phenotype of dendritic cells. Transplantation 2007, 84, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Murakami, T.; Oppenheim, J.J.; Howard, O.M. Triptolide, a constituent of immunosuppressive Chinese herbal medicine, is a potent suppressor of dendritic-cell maturation and trafficking. Blood 2005, 106, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.J.; Shen, Q.Y.; Cheng, H.; Mao, X.H.; Lao, L.M.; Hao, G.L. Triptolide affects the differentiation, maturation and function of human dendritic cells. Int. Immunopharmacol. 2005, 5, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.H.; Shang, P.Z.; Lu, Q.J.; Wu, X. Triptolide regulates T cell-mediated immunity via induction of CD11c(low) dendritic cell differentiation. Food Chem. Toxicol. 2012, 50, 2560–2564. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, T.; Chen, G.; Li, N.; Wang, J.; Ma, P.; Cao, X. Immunosuppressant triptolide inhibits dendritic cell-mediated chemoattraction of neutrophils and T cells through inhibiting Stat3 phosphorylation and NF-κB activation. Biochem. Biophys. Res. Commun. 2006, 345, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H.; Panayi, G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Okamoto, H.; Toyama, Y.; Momohara, S. Molecular aspects of rheumatoid arthritis: Chemokines in the joints of patients. FEBS J. 2008, 275, 4448–4455. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.U.; Kwok, S.K.; Hong, K.H.; Yoo, S.A.; Kong, J.S.; Choe, J.; Cho, C.S. Soluble Fas ligand inhibits angiogenesis in rheumatoid arthritis. Arthritis Res. Ther. 2007, 9, R42. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.D., Jr. Rheumatoid arthritis. Pathophysiology and implications for therapy. N. Engl. J. Med. 1990, 322, 1277–1289. [Google Scholar] [PubMed]
- Sivalingam, S.P.; Thumboo, J.; Vasoo, S.; Thio, S.T.; Tse, C.; Fong, K.Y. In vivo pro- and anti-inflammatory cytokines in normal and patients with rheumatoid arthritis. Ann. Acad. Med. Singap. 2007, 36, 96–99. [Google Scholar] [PubMed]
- Koch, A.E. Angiogenesis as a target in rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, ii60–ii67. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhou, J.; He, Y.; Jia, H.; Zhao, L.; Zhao, N.; Lu, A. Effects of triptolide from Radix Tripterygium wilfordii (Leigongteng) on cartilage cytokines and transcription factor NF-κB: A study on induced arthritis in rats. Chin. Med. 2009, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, V.; Dey, M.; Dorn, R.; Raskin, I. MyD88-dependent and independent pathways of Toll-Like Receptors are engaged in biological activity of Triptolide in ligand-stimulated macrophages. BMC Chem. Biol. 2010, 10, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yifan, W.; Dengming, W.; Zheng, L.; Yanping, L.; Junkan, S. Triptolide inhibits CCR5 expressed in synovial tissue of rat adjuvant-induced arthritis. Pharmacol. Rep. 2007, 59, 795–799. [Google Scholar] [PubMed]
- Wang, Y.; Wei, D.; Lai, Z.; Le, Y. Triptolide inhibits CC chemokines expressed in rat adjuvant-induced arthritis. Int. Immunopharmacol. 2006, 6, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Liu, C.; Xiao, C.; Jia, H.; Imada, K.; Wu, H.; Ito, A. Triptolide, a diterpenoid triepoxide, suppresses inflammation and cartilage destruction in collagen-induced arthritis mice. Biochem. Pharmacol. 2007, 73, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, L.; Tao, X.; Lipsky, P.E. Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthritis Rheum. 2004, 50, 2995–3003. [Google Scholar] [CrossRef] [PubMed]
- Bleharski, J.R.; Kiessler, V.; Buonsanti, C.; Sieling, P.A.; Stenger, S.; Colonna, M.; Modlin, R.L. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J. Immunol. 2003, 170, 3812–3818. [Google Scholar] [CrossRef] [PubMed]
- Bouchon, A.; Dietrich, J.; Colonna, M. Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 2000, 164, 4991–4995. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.T.; Kang, E.J.; Ha, Y.J.; Song, J.S. Levels of plasma-soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) are correlated with disease activity in rheumatoid arthritis. J. Rheumatol. 2012, 39, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Fortin, C.F.; Lesur, O.; Fulop, T., Jr. Effects of TREM-1 activation in human neutrophils: Activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int. Immunol. 2007, 19, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Sun, H.; Ao, M.; Zhao, C. Atomic Force Microscopy Study of the Anti-inflammatory Effects of Triptolide on Rheumatoid Arthritis Fibroblast-like Synoviocytes. Microsc. Microanal. 2017, 23, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, Y.; Qiu, Q.; Xiao, Y.; Huang, M.; Shi, M.; Liang, L.; Yang, X.; Xu, H. Triptolide inhibits the migration and invasion of rheumatoid fibroblast-like synoviocytes by blocking the activation of the JNK MAPK pathway. Int. Immunopharmacol. 2016, 41, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Liacini, A.; Sylvester, J.; Zafarullah, M. Triptolide suppresses proinflammatory cytokine-induced matrix metalloproteinase and aggrecanase-1 gene expression in chondrocytes. Biochem. Biophys. Res. Commun. 2005, 327, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Sato, T.; Ito, A. Triptolide, a novel diterpenoid triepoxide from Tripterygium wilfordii Hook. f., suppresses the production and gene expression of pro-matrix metalloproteinases 1 and 3 and augments those of tissue inhibitors of metalloproteinases 1 and 2 in human synovial fibroblasts. Arthritis Rheum. 2001, 44, 2193–2200. [Google Scholar] [PubMed]
- Lu, Y.; Wang, W.J.; Leng, J.H.; Cheng, L.F.; Feng, L.; Yao, H.P. Inhibitory effect of triptolide on interleukin-18 and its receptor in rheumatoid arthritis synovial fibroblasts. Inflamm. Res. 2008, 57, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Van Roon, J.A.; van Roy, J.L.; Gmelig-Meyling, F.H.; Lafeber, F.P.; Bijlsma, J.W. Prevention and reversal of cartilage degradation in rheumatoid arthritis by interleukin-10 and interleukin-4. Arthritis Rheum. 1996, 39, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, E.; Kuroda, A.; Taki, H.; Ikemoto, M.; Hori, T.; Yamashita, N.; Maruyama, M.; Kobayashi, M. Interleukin 10 cooperates with interleukin 4 to suppress inflammatory cytokine production by freshly prepared adherent rheumatoid synovial cells. J. Rheumatol. 1995, 22, 2020–2026. [Google Scholar] [PubMed]
- Szekanecz, Z.; Koch, A.E. Vascular involvement in rheumatic diseases: “vascular rheumatology”. Arthritis Res. Ther. 2008, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Lainer-Carr, D.; Brahn, E. Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat. Clin. Pract. Rheumatol. 2007, 3, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.E. Review: Angiogenesis: Implications for rheumatoid arthritis. Arthritis Rheum. 1998, 41, 951–962. [Google Scholar] [CrossRef]
- Veale, D.J.; Fearon, U. Inhibition of angiogenic pathways in rheumatoid arthritis: Potential for therapeutic targeting. Best Pract. Res. Clin. Rheumatol. 2006, 20, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, Y.; Liu, C.; Guo, W.; Li, X.; Su, X.; Wan, H.; Sun, Y.; Lin, N. Anti-angiogenic effect of triptolide in rheumatoid arthritis by targeting angiogenic cascade. PLoS ONE 2013, 8, e77513. [Google Scholar] [CrossRef] [PubMed]
- He, M.F.; Huang, Y.H.; Wu, L.W.; Ge, W.; Shaw, P.C.; But, P.P. Triptolide functions as a potent angiogenesis inhibitor. Int. J. Cancer 2010, 126, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Y.; Kong, X.; Zhu, L.; Pang, J.; Xu, Y.; Chen, W.; Zhan, H.; Lu, A.; Lin, N. Triptolide Prevents Bone Destruction in the Collagen-Induced Arthritis Model of Rheumatoid Arthritis by Targeting RANKL/RANK/OPG Signal Pathway. Evid. Based Complement. Altern. Med. 2013, 2013, 626038. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.Y.; Wang, L.; Sun, C.; Li, D.J. Estrogen enhances the functions of CD4+CD25+Foxp3+ regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro. Cell. Mol. Immunol. 2011, 8, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.P.; Wang, G.D.; Du, X.J.; Wan, G.; Wu, J.T.; Miao, L.B.; Liang, Q.D. Triptolide inhibits the function of TNF-α in osteoblast differentiation by inhibiting the NF-κB signaling pathway. Exp. Ther. Med. 2017, 14, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Cooles, F.A.; Isaacs, J.D. Pathophysiology of rheumatoid arthritis. Curr. Opin. Rheumatol. 2011, 23, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Noss, E.H.; Brenner, M.B. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol. Rev. 2008, 223, 252–270. [Google Scholar] [CrossRef] [PubMed]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, N.; Yamazaki, R.; Kitasato, H.; Beppu, M.; Aoki, H.; Kawai, S. Triptolide, an active compound identified in a traditional Chinese herb, induces apoptosis of rheumatoid synovial fibroblasts. BMC Pharmacol. 2004, 4, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, X.; Cui, J.; Wu, Y.; Han, X.; Gao, C.; Hua, Z.; Shen, P. The roles of endogenous reactive oxygen species and nitric oxide in triptolide-induced apoptotic cell death in macrophages. J. Mol. Med. 2007, 85, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Bai, X.J.; Hu, D.; Li, Z.F.; Liu, K.J. Effect of triptolide on secretion of inflammatory cellular factors TNF-α and IL-8 in peritoneal macrophages of mice activated by lipopolysaccharide. World J. Emerg. Med. 2010, 1, 70–74. [Google Scholar] [PubMed]
- Wang, X.; Jiang, Z.; Cao, W.; Yuan, Z.; Sun, L.; Zhang, L. Th17/Treg imbalance in triptolide-induced liver injury. Fitoterapia 2014, 93, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, F.; Guan, C.; Wang, W.; Sun, X.; Fu, X.; Huang, M.; Jin, J.; Huang, Z. Activation of Nrf2 protects against triptolide-induced hepatotoxicity. PLoS ONE 2014, 9, e100685. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jiang, F.; Lu, A.; Zhang, G. Linkers Having a Crucial Role in Antibody-Drug Conjugates. Int. J. Mol. Sci. 2016, 17, 561. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Qiu, Y.; Xu, H.; Ao, W.; Lam, W.; Yang, X. Acute and subacute toxicity studies on triptolide and triptolide-loaded polymeric micelles following intravenous administration in rodents. Food Chem. Toxicol. 2013, 57, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Huang, X.; Shu, B.; Xue, M.; Zhang, P.; Wang, T.; Liu, L.; Jiang, Z.; Zhang, L. Inhibition of mitochondrial respiratory chain is involved in triptolide-induced liver injury. Fitoterapia 2011, 82, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, L.; Wang, L.; Hassan, H.M.; Wang, X.; Hylemon, P.B.; Wang, T.; Zhou, H.; Zhang, L.; Jiang, Z. Activation of Sirt1/FXR Signaling Pathway Attenuates Triptolide-Induced Hepatotoxicity in Rats. Front. Pharmacol. 2017, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xie, T.; Zhang, Y.; Zhou, F.; Ruan, J.; Zhu, W.; Zhu, H.; Feng, Z.; Zhou, X. Triptolide Induces hepatotoxicity via inhibition of CYP450s in Rat liver microsomes. BMC Complement. Altern. Med. 2017, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ren, L.; Zhuo, L.; Ananda, S.; Liu, L. Involvement of oxidative stress in the mechanism of triptolide-induced acute nephrotoxicity in rats. Exp. Toxicol. Pathol. 2012, 64, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhuo, L.; Ananda, S.; Sun, T.; Li, S.; Liu, L. Role of reactive oxygen species in triptolide-induced apoptosis of renal tubular cells and renal injury in rats. J. Huazhong Univ. Sci. Technol. 2011, 31, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, Z.; Liu, L.; Zhang, Y.; Zhang, S.; Xiao, J.; Ma, M.; Zhang, L. Triptolide induces adverse effect on reproductive parameters of female Sprague-Dawley rats. Drug Chem. Toxicol. 2011, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Jiang, Z.; Huang, X.; Xu, F.; Zhang, R.; Zhang, Z.; Tian, Y.; Wang, T.; Zhu, T.; Liu, J.; et al. Male Reproductive Toxicity and Toxicokinetics of Triptolide in Rats. Arzneimittelforschung 2008, 58, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, L.; Mu, X.; Jiang, Z.; Zhang, L. Effect of triptolide on estradiol release from cultured rat granulosa cells. Endocr. J. 2012, 59, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, Z.; Mu, X.; Wen, J.; Su, Y.; Zhang, L. Effect of triptolide on progesterone production from cultured rat granulosa cells. Arzneimittelforschung 2012, 62, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Hu, S.; Elgehama, A.; Shao, F.; Ren, R.; Liu, W.; Zhang, W.; Wang, X.; Tan, R.; Xu, Q.; et al. Fumigaclavine C ameliorates dextran sulfate sodium-induced murine experimental colitis via NLRP3 inflammasome inhibition. J. Pharmacol. Sci. 2015, 129, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, G.Z.; Zheng, N.; Liu, L. Injury of myocadium of rats by acute triptolide poisoning. ICI World J. 2010, 24, 460–465. [Google Scholar]
- Zhang, C.; Gu, C.; Peng, F.; Liu, W.; Wan, J.; Xu, H.; Lam, W.C.; Yang, X. Preparation and Optimization of Triptolide-Loaded Solid Lipid Nanoparticles for Oral Delivery with Reduced Gastric Irritation. Molecules 2013, 18, 13340–13356. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Gong, L.; Qi, X.; Wu, Y.; Xing, G.; Yao, J.; Luan, Y.; Xiao, Y.; Li, Y.; Wu, X.; et al. Knockout of hepatic P450 reductase aggravates triptolide-induced toxicity. Toxicol. Lett. 2011, 205, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jiang, Z.; Liu, J.; Huang, X.; Wang, T.; Liu, J.; Zhang, Y.; Zhou, Z.; Guo, J.; Yang, L.; et al. Sex differences in subacute toxicity and hepatic microsomal metabolism of triptolide in rats. Toxicology 2010, 271, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q. Triptolide and its expanding multiple pharmacological functions. Int. Immunopharmacol. 2011, 11, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.; Huang, X.; Su, Y.; Ji, J.; Su, Y.; Jiang, Z.; Zhang, L. Glycyrrhizin accelerates the metabolism of triptolide through induction of CYP3A in rats. J. Ethnopharmacol. 2014, 152, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, P.P.; Guo, H.T.; Liu, Y.; Li, J.; He, X.J.; Lu, A.P. Corrigendum: Safety Profiles of Tripterygium wilfordii Hook F: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2017, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Y.; Fu, L.; Li, Y.; Lou, L. (5R)-5-hydroxytriptolide (LLDT-8), a novel immunosuppressant in clinical trials, exhibits potent antitumor activity via transcription inhibition. Cancer Lett. 2012, 324, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Fidler, J.M.; Li, K.; Chung, C.; Wei, K.; Ross, J.A.; Gao, M.; Rosen, G.D. PG490-88, a derivative of triptolide, causes tumor regression and sensitizes tumors to chemotherapy. Mol. Cancer Ther. 2003, 2, 855–862. [Google Scholar] [PubMed]
- Wu, D.D.; Huang, L.; Zhang, L.; Wu, L.-Y.; Li, Y.-C.; Feng, L. LLDT-67 attenuates MPTP-induced neurotoxicity in mice by up-regulating NGF expression. Acta Pharmacol. Sin. 2012, 33, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fan, X.; Zhang, G.; Liu, X.; Li, Z.; Li, Y.; Jiang, B. LLDT-288, a novel triptolide analogue exhibits potent antitumor activity in vitro and in vivo. Biomed. Pharmacother. 2017, 93, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zuo, J.P. Immunosuppressant discovery from Tripterygium wilfordii Hook f: The novel triptolide analog (5R)-5-hydroxytriptolide (LLDT-8). Acta Pharmacol. Sin. 2012, 33, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tang, W.; Ren, Y.-X.; He, P.-L.; Zhang, F.; Shi, L.-P.; Fu, Y.-F.; Li, Y.-C.; Ono, S.; Fujiwara, H.; et al. (5R)-5-hydroxytriptolide attenuated collagen-induced arthritis in DBA/1 mice via suppressing interferon-γ production and its related signaling. J. Pharmacol. Exp. Ther. 2006, 318, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tang, W.; He, P.-L.; Li, X.-Y.; Yang, Y.-F.; Li, Y.-C.; Geng, J.-G.; Zuo, J.-P. Inhibition of inducible nitric-oxide synthase expression by (5R)-5-hydroxytriptolide in interferon-γ- and bacterial lipopolysaccharide-stimulated macrophages. J. Pharmacol. Exp. Ther. 2006, 316, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.F.; Ni, J.; Zhong, X.-G.; Tang, W.; Zhou, R.; Zhou, Y.; Dong, J.-R.; He, P.-L.; Wan, H.; Li, Y.-C.; et al. (5R)-5-hydroxytriptolide (LLDT-8), a novel triptolide derivative, prevents experimental autoimmune encephalomyelitis via inhibiting T cell activation. J. Neuroimmunol. 2006, 175, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Modi, S.; McGinn, O.; Zhao, X.; Dudeja, V.; Ramakrishnan, S.; Saluja, A.K. Impaired Synthesis of Stromal Components in Response to Minnelide Improves Vascular Function, Drug Delivery, and Survival in Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.Z.; Shi, Y.; Fidler, J.M.; Chen, R.; Ling, X.; Plunkett, W.; Andreeff, M. MRx102, a triptolide derivative, has potent antileukemic activity in vitro and in a murine model of AML. Leukemia 2012, 26, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chen, C.H.; Lin, Z.C.; Fang, J.Y. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J. Food Drug Anal. 2017, 25, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hao, B.; Ju, D.; Liu, M.; Zhao, H.; Du, Z.; Xia, J. Pharmacokinetic and pharmacodynamic study of triptolide-loaded liposome hydrogel patch under microneedles on rats with collagen-induced arthritis. Acta Pharm. Sin. B 2015, 5, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, T.; Li, Q.; Huang, J.; Xu, H.; Li, J.; Wang, Y.; Liang, Q. Fabrication of novel vesicles of triptolide for antirheumatoid activity with reduced toxicity in vitro and in vivo. Int. J. Nanomed. 2016, 11, 2663–2673. [Google Scholar]
- Liu, M.; Dong, J.; Yang, Y.; Yang, X.; Xu, H. Anti-inflammatory effects of triptolide loaded poly(d,l-lactic acid) nanoparticles on adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2005, 97, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Dong, J.; Yang, Y.; Yang, X.; Xu, H. Effect of poly(d,l-lactic acid) nanoparticles as triptolide carrier on abating rats renal toxicity by NMR-based metabolic analysis. J. Nanosci. Nanotechnol. 2008, 8, 3493–3499. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Wu, Q.; Hu, S.; Li, X.; Yang, X. Triptolide loaded solid lipid nanoparticle hydrogel for topical application. Drug Dev. Ind. Pharm. 2005, 31, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Chen, H.; Weng, T.; Yang, Y.; Yang, X. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur. J. Pharm. Biopharm. 2003, 56, 189–196. [Google Scholar] [CrossRef]
- Mei, Z.; Li, X.; Wu, Q.; Hu, S.; Yang, X. The research on the anti-inflammatory activity and hepatotoxicity of triptolide-loaded solid lipid nanoparticle. Pharmacol. Res. 2005, 51, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zhao, Y.; Li, X.-J.; Jiang, Z.-Z.; Zhang, L.; Liu, S.-H.; Li, X.-M.; Zhang, L.-Y.; Yang, S.-Y. Comparison of toxicokinetic and tissue distribution of triptolide-loaded solid lipid nanoparticles vs free triptolide in rats. Eur. J. Pharm. Sci. 2012, 47, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Peng, F.; Liu, W.; Wan, J.; Wan, C.; Xu, H.; Lam, C.W.; Yang, X. Nanostructured lipid carriers as a novel oral delivery system for triptolide: Induced changes in pharmacokinetics profile associated with reduced toxicity in male rats. Int. J. Nanomed. 2014, 9, 1049–1063. [Google Scholar]
- Xu, L.; Pan, J.; Chen, Q.; Yu, Q.; Chen, H.; Xu, H.; Qiu, Y.; Yang, X. In vivo evaluation of the safety of triptolide-loaded hydrogel-thickened microemulsion. Food Chem. Toxicol. 2008, 46, 3792–3799. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mader, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- zur Muhlen, A.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery—Drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chang, X.; Weng, T.; Zhao, X.; Gao, Z.; Yang, Y.; Xu, H.; Yang, X. A study of microemulsion systems for transdermal delivery of triptolide. J. Control Release 2004, 98, 427–436. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Lu, C.; Zheng, G.; He, X.; Wang, M.; Chen, G.; Zhang, G.; Lu, A. Combination therapeutics in complex diseases. J. Cell. Mol. Med. 2016, 20, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Li, W.; Yan, Y.; Mao, C.; Cai, R.; Xu, H.; Yang, X. Effects of cytochrome P4503A inducer dexamethasone on the metabolism and toxicity of triptolide in rat. Toxicol. Lett. 2010, 192, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Ding, W.; Jia, R.; Du, J.; Wang, T.; Zhang, C.; Gu, Z.; Yin, G. Anti-inflammatory and hepatoprotective effects of glycyrrhetinic acid on CCl4-induced damage in precision-cut liver slices from Jian carp (Cyprinus carpio var. jian) through inhibition of the NF-κB pathway. Fish Shellfish Immunol. 2017, 64, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Kao, P.N. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D 2003, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.J. Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk. Lymphoma 2001, 42, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ding, L.; Qiu, F. Potential drug interactions associated with glycyrrhizin and glycyrrhetinic acid. Drug Metab. Rev. 2015, 47, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Wang, G.; Xie, H.; Zhu, X.; Sun, J.; A, J. Pharmacokinetic study of triptolide, a constituent of immunosuppressive chinese herb medicine, in rats. Biol. Pharm. Bull. 2007, 30, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.-L.; zhuang, X.-M.; Yang, H.-Y.; Yuan, M.; Xu, L.; Li, H. Inhibition of P-glycoprotein Gene Expression and Function Enhances Triptolide-induced Hepatotoxicity in Mice. Sci. Rep. 2015, 5, 11747. [Google Scholar] [CrossRef] [PubMed]
- Han, F.M.; Peng, Z.H.; Wang, J.J.; Chen, Y. In vivo effect of triptolide combined with glycyrrhetinic acid on rat cytochrome P450 enzymes. Yao Xue Xue Bao 2013, 48, 1136–1141. [Google Scholar] [PubMed]
- Li, Z.; Yan, M.; Cao, L.; Fang, P.; Guo, Z.; Hou, Z.; Zhang, B. Glycyrrhetinic Acid Accelerates the Clearance of Triptolide through P-gp In Vitro. Phytother. Res. 2017, 31, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Arafa, H.M. Uroprotective effects of curcumin in cyclophosphamide-induced haemorrhagic cystitis paradigm. Basic Clin. Pharmacol. Toxicol. 2009, 104, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, Q.H.; Li, Y.X.; Huang, Y.F.; Xie, J.H.; Xu, L.Q.; Dou, Y.X.; Su, Z.R.; Zeng, H.F.; Chen, J.N. Protective effects of silymarin on triptolide-induced acute hepatotoxicity in rats. Mol. Med. Rep. 2018, 17, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.X.; Jia, L.; Lim, L.Y.; Lin, J.C.; Shu, G.; Zhao, L.; Ye, G.; Liang, X.X.; Ji, H.; Fu, H.L. Renal-targeted delivery of triptolide by entrapment in pegylated TRX-20-modified liposomes. Int. J. Nanomed. 2017, 12, 5673–5686. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.X.; Wu, X.J.; Mo, J.; Wang, Y.L.; Xu, C.Q.; Lim, L.Y. Renal targeted delivery of triptolide by conjugation to the fragment peptide of human serum albumin. Eur. J. Pharm. Biopharm. 2015, 94, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lin, Q.; Gong, T.; Sun, X.; Zhang, Z.R. Renal-targeting triptolide-glucosamine conjugate exhibits lower toxicity and superior efficacy in attenuation of ischemia/reperfusion renal injury in rats. Acta Pharmacol. Sin. 2016, 37, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, Q.; Han, J.; Gao, G.; Liu, J.; Gong, T.; Gu, Z.; Huang, Y.; Sun, X.; He, Q. The targeting of 14-succinate triptolide-lysozyme conjugate to proximal renal tubular epithelial cells. Biomaterials 2009, 30, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wong, B.C.K.; Chen, H.; Bian, Z.; Zhang, G.; Zhang, X.; Kashif Riaz, M.; Tyagi, D.; Lin, G.; Zhang, Y.; et al. Pulmonary delivery of triptolide-loaded liposomes decorated with anti-carbonic anhydrase IX antibody for lung cancer therapy. Sci. Rep. 2017, 7, 1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, B.; Xu, X.; Zhuang, B.; Li, H.; Yin, J.; Cong, M.; Xu, W.; Lu, A. Toward targeted therapy in chemotherapy-resistant pancreatic cancer with a smart triptolide nanomedicine. Oncotarget 2016, 7, 8360–8372. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.; Xia, H.; Park, W.; Hackett, M.J.; Song, C.; Na, K.; Hui, K.M.; Hyeon, T. pH-sensitive nanoformulated triptolide as a targeted therapeutic strategy for hepatocellular carcinoma. ACS Nano 2014, 8, 8027–8039. [Google Scholar] [CrossRef] [PubMed]


| No. | Compound Name | Chemical Structure | Modification Sites | Improved Characteristics Compared with TP | References |
|---|---|---|---|---|---|
| 1 | (5R)-5-hydroxytriptolide (LLDT-8) |  | C-5 site | much lower toxicity | [88] |
| 2 | LLDT-67 |  | C-14 site | low toxicity | [86] |
| 3 | LLDT-288 |  | C-14 site | low toxicity | [87] |
| 4 | PG490-88 |  | C-14-hydroxyl site | Water soluble | [85] |
| 5 | Minnelide |  | C-14-hydroxyl site | Water soluble | [92] |
| 6 | MRx102 | —— | —— | low toxicity | [93] |
| Drug Carrier | In Vivo/In Vitro | Advantages | References |
|---|---|---|---|
| liposome hydrogel patch | CIA rats | improves bioavailability of TP; bypasses hepatic first-pass metabolism, and reduces the incidence or severity of gastrointestinal reactions | [96] |
| nanodrug carrier system (γ-PGA-l-PAE-TP (PPT)) | normal C57/B6 mice/RAW264.7 cell lines | reduces free TP toxicity in vitro and in vivo | [97] |
| poly(d,l-lactic acid) (PLA) nanoparticles | AIA rats | improve bioavailability of TP | [98] |
| poly(d,l-lactic acid) (PLA) nanoparticles | normal SD rats | abate the renal toxicity caused by TP | [99] |
| solid lipid nanoparticle hydrogel | carrageenan-induced rats | improves safety and minimizes the toxicity induced by TP | [100] |
| solid lipid nanoparticle/microemulsions | carrageenan-induced rats and AIA rats | increase therapeutic index | [101] |
| solid lipid nanoparticles | carrageenan-induced rats | enhance the anti-inflammatory activity of TP have a protective effect against TP-induced hepatotoxicity | [102] |
| solid lipid nanoparticles | normal SD rats | reduce gastric irritation | [78] |
| solid lipid nanoparticles | normal SD rats | enhance efficacy, decrease reproductive toxicity | [103] |
| nanostructured lipid carriers | normal SD rats | reduce subacute toxicity in male rats | [104] |
| hydrogel-thickened microemulsion | normal rabbits, mice, beagle dogs, guinea pigs | no obvious toxicities | [105] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, D.; Guo, Q.; Shen, J.; Zheng, K.; Lu, C.; Zhang, G.; Lu, A.; He, X. The Effect of Triptolide in Rheumatoid Arthritis: From Basic Research towards Clinical Translation. Int. J. Mol. Sci. 2018, 19, 376. https://doi.org/10.3390/ijms19020376
Fan D, Guo Q, Shen J, Zheng K, Lu C, Zhang G, Lu A, He X. The Effect of Triptolide in Rheumatoid Arthritis: From Basic Research towards Clinical Translation. International Journal of Molecular Sciences. 2018; 19(2):376. https://doi.org/10.3390/ijms19020376
Chicago/Turabian StyleFan, Danping, Qingqing Guo, Jiawen Shen, Kang Zheng, Cheng Lu, Ge Zhang, Aiping Lu, and Xiaojuan He. 2018. "The Effect of Triptolide in Rheumatoid Arthritis: From Basic Research towards Clinical Translation" International Journal of Molecular Sciences 19, no. 2: 376. https://doi.org/10.3390/ijms19020376
APA StyleFan, D., Guo, Q., Shen, J., Zheng, K., Lu, C., Zhang, G., Lu, A., & He, X. (2018). The Effect of Triptolide in Rheumatoid Arthritis: From Basic Research towards Clinical Translation. International Journal of Molecular Sciences, 19(2), 376. https://doi.org/10.3390/ijms19020376