The Effects of the WNT-Signaling Modulators BIO and PKF118-310 on the Chondrogenic Differentiation of Human Mesenchymal Stem Cells
Abstract
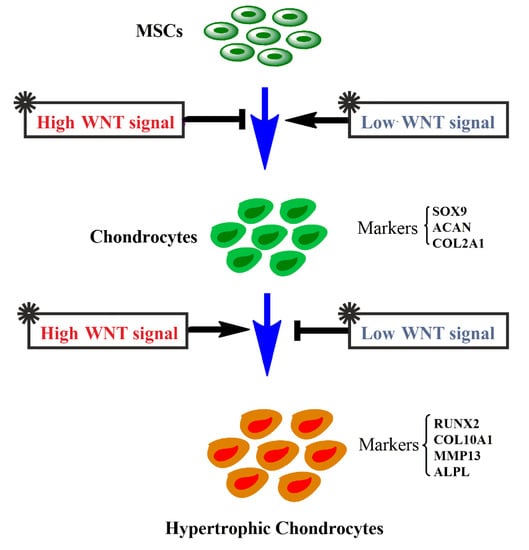
:1. Introduction
2. Results
2.1. The Efficacy and Specificity of BIO and PKF on the TCF/LEF Reporter
2.2. The Effects of Activation and Inhibition of Canonical WNT Signaling on Gene Expression during hMSCs Chondrogenesis
2.3. High Levels of Canonical WNT Signaling Inhibited Cartilage Formation
2.4. BIO Significantly Decreased the Number of Apoptotic Cells, While PKF Induced hMSCs Apoptosis in Pellets
2.5. The Protein Level of DKK1 and MMP1 Was Decreased in Pellet Culture Medium with PKF Treatment
3. Discussion
4. Materials and Methods
4.1. Luciferase Assay
4.2. Cell Culture and Expansion
4.3. Pellet Culture and Chondrogenic Differentiation of hMSCs
4.4. RNA Isolation and Quantitative PCR
4.5. Alcian Blue/Safranin O and Alizarin Red Staining
4.6. Immunofluorescent (IF) Staining for Collagen Type II
4.7. Immunohistochemistry (IHC) of β-Catenin and Collagen Type X
4.8. TUNEL Assay
4.9. GAG and DNA Assay
4.10. Enzyme Linked Immunosorbent Assay (ELISA)
4.11. Statistical Analysis
Author Contributions
Conflicts of Interest
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Worster, A.A.; Brower-Toland, B.D.; Fortier, L.A.; Bent, S.J.; Williams, J.; Nixon, A.J. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2001, 19, 738–749. [Google Scholar] [CrossRef]
- Noth, U.; Steinert, A.F.; Tuan, R.S. Technology insight: Adult mesenchymal stem cells for osteoarthritis therapy. Nat. Clin. Pract. Rheumatol. 2008, 4, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R. The Wnts. Genome Biol. 2002, 3, Reviews3001. [Google Scholar] [PubMed]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Hocking, A.M.; Brown, J.D.; Moon, R.T. Mechanism and function of signal transduction by the Wnt/β-catenin and Wnt/Ca2+ pathways. Oncogene 1999, 18, 7860–7872. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Neth, P.; Ciccarella, M.; Egea, V.; Hoelters, J.; Jochum, M.; Ries, C. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells 2006, 24, 1892–1903. [Google Scholar] [CrossRef] [PubMed]
- Kirton, J.P.; Crofts, N.J.; George, S.J.; Brennan, K.; Canfield, A.E. Wnt/β-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: Potential relevance to vascular disease? Circ. Res. 2007, 101, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Hoppler, S.; Kavanagh, C.L. Wnt signalling: Variety at the core. J. Cell Sci. 2007, 120 Pt 3, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Tran, F.H.; Zheng, J.J. Modulating the wnt signaling pathway with small molecules. Protein Sci. Publ. Protein Soc. 2017, 26, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, R.; Yuasa, T.; Williams, J.A.; Byers, S.W.; Shah, S.; Pacifici, M.; Iwamoto, M.; Enomoto-Iwamoto, M. Wnt/β-catenin and retinoic acid receptor signaling pathways interact to regulate chondrocyte function and matrix turnover. J. Biol. Chem. 2010, 285, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Lepourcelet, M.; Chen, Y.N.; France, D.S.; Wang, H.; Crews, P.; Petersen, F.; Bruseo, C.; Wood, A.W.; Shivdasani, R.A. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell 2004, 5, 91–102. [Google Scholar] [CrossRef]
- Wei, W.; Chua, M.S.; Grepper, S.; So, S. Small molecule antagonists of Tcf4/β-catenin complex inhibit the growth of HCC cells in vitro and in vivo. Int. J. Cancer 2010, 126, 2426–2436. [Google Scholar]
- Gandhirajan, R.K.; Staib, P.A.; Minke, K.; Gehrke, I.; Plickert, G.; Schlosser, A.; Schmitt, E.K.; Hallek, M.; Kreuzer, K.A. Small molecule inhibitors of Wnt/beta-catenin/lef-1 signaling induces apoptosis in chronic lymphocytic leukemia cells in vitro and in vivo. Neoplasia 2010, 12, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Zhou, F.Q.; Zhou, J.; Yokota, Y.; Wang, Y.M.; Yoshimura, T.; Kaibuchi, K.; Woodgett, J.R.; Anton, E.S.; Snider, W.D. Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron 2006, 52, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chu, Y.; Lv, X.; Qiu, P.; Liu, C.; Zhang, H.; Li, D.; Peng, S.; Dou, Z.; Hua, J. GSK3 inhibitor-BIO regulates proliferation of immortalized pancreatic mesenchymal stem cells (iPMSCs). PLoS ONE 2012, 7, e31502. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Bai, Y.; Chu, Z.; Wang, J.; Wang, L.; Yu, M.; Lian, Z.; Hua, J. GSK3 inhibitor-BIO regulates proliferation of female germline stem cells from the postnatal mouse ovary. Cell Prolif. 2012, 45, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.S.; Engel, F.B.; Keating, M.T. The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chem. Biol. 2006, 13, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Meijer, L.; Skaltsounis, L.; Greengard, P.; Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Baghaban Eslaminejad, M.; Fallah, N. Small Molecule-BIO Accelerates and Enhances Marrow-Derived Mesenchymal Stem Cell in Vitro Chondrogenesis. Iran. J. Med. Sci. 2014, 39, 107–116. [Google Scholar] [PubMed]
- Landman, E.B.; Miclea, R.L.; van Blitterswijk, C.A.; Karperien, M. Small molecule inhibitors of WNT/beta-catenin signaling block IL-1beta- and TNFalpha-induced cartilage degradation. Arthritis Res. Ther. 2013, 15, R93. [Google Scholar] [CrossRef] [PubMed]
- Leow, P.C.; Tian, Q.; Ong, Z.Y.; Yang, Z.; Ee, P.L. Antitumor activity of natural compounds, curcumin and PKF118-310, as Wnt/beta-catenin antagonists against human osteosarcoma cells. Investig. New Drugs 2010, 28, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Hallett, R.M.; Kondratyev, M.K.; Giacomelli, A.O.; Nixon, A.M.; Girgis-Gabardo, A.; Ilieva, D.; Hassell, J.A. Small molecule antagonists of the Wnt/β-catenin signaling pathway target breast tumor-initiating cells in a Her2/Neu mouse model of breast cancer. PLoS ONE 2012, 7, e33976. [Google Scholar] [CrossRef] [PubMed]
- Leijten, J.C.; Emons, J.; Sticht, C.; van Gool, S.; Decker, E.; Uitterlinden, A.; Rappold, G.; Hofman, A.; Rivadeneira, F.; Scherjon, S.; et al. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 2012, 64, 3302–3312. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.G.; Ryu, J.H.; Kim, I.C.; Jho, E.H.; Jung, H.C.; Kim, K.; Kim, S.J.; Chun, J.S. Wnt-7a causes loss of differentiated phenotype and inhibits apoptosis of articular chondrocytes via different mechanisms. J. Biol. Chem. 2004, 279, 26597–26604. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhong, L.; van Blitterswijk, C.A.; Post, J.N.; Karperien, M. T cell factor 4 is a pro-catabolic and apoptotic factor in human articular chondrocytes by potentiating nuclear factor kappaB signaling. J. Biol. Chem. 2013, 288, 17552–17558. [Google Scholar] [CrossRef] [PubMed]
- Nalesso, G.; Thomas, B.L.; Sherwood, J.C.; Yu, J.; Addimanda, O.; Eldridge, S.E.; Thorup, A.S.; Dale, L.; Schett, G.; Zwerina, J.; et al. WNT16 antagonises excessive canonical WNT activation and protects cartilage in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Tang, D.; Wu, Q.; Hao, S.; Chen, M.; Xie, C.; Rosier, R.N.; O’Keefe, R.J.; Zuscik, M.; Chen, D. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2009, 24, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lories, R.J.; Peeters, J.; Bakker, A.; Tylzanowski, P.; Derese, I.; Schrooten, J.; Thomas, J.T.; Luyten, F.P. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 2007, 56, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Freyria, A.M.; Mallein-Gerin, F. Chondrocytes or adult stem cells for cartilage repair: The indisputable role of growth factors. Injury 2012, 43, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Puetzer, J.L.; Petitte, J.N.; Loboa, E.G. Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng. Part B Rev. 2010, 16, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, R.; Ohta, Y.; Yuasa, T.; Kondo, N.; Hoang, T.; Addya, S.; Fortina, P.; Pacifici, M.; Iwamoto, M.; Enomoto-Iwamoto, M. Roles of beta-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab. Investig. J. Tech. Methods Pathol. 2011, 91, 1739–1752. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.B.; Brockbank, S.M.; van Lent, P.L.; van Beuningen, H.M.; Geurts, J.; Takahashi, N.; van der Kraan, P.M.; van de Loo, F.A.; Schreurs, B.W.; Clements, K.; et al. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: Prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009, 60, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Huang, X.; Karperien, M.; Post, J.N. The Regulatory Role of Signaling Crosstalk in Hypertrophy of MSCs and Human Articular Chondrocytes. Int. J. Mol. Sci. 2015, 16, 19225–19247. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Huang, X.; Rodrigues, E.D.; Leijten, J.C.; Verrips, T.; El Khattabi, M.; Karperien, M.; Post, J.N. Endogenous DKK1 and FRZB Regulate Chondrogenesis and Hypertrophy in Three-Dimensional Cultures of Human Chondrocytes and Human Mesenchymal Stem Cells. Stem Cells Dev. 2016, 25, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; van Blitterswijk, C.A.; Karperien, M. A Wnt/beta-catenin negative feedback loop inhibits interleukin-1-induced matrix metalloproteinase expression in human articular chondrocytes. Arthritis Rheum. 2012, 64, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Schivo, S.; Huang, X.; Leijten, J.; Karperien, M.; Post, J.N. Nitric Oxide Mediates Crosstalk between Interleukin 1beta and WNT Signaling in Primary Human Chondrocytes by Reducing DKK1 and FRZB Expression. Int. J. Mol. Sci. 2017, 18, 2491. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Manner, P.A.; Horner, A.; Shum, L.; Tuan, R.S.; Nuckolls, G.H. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2004, 12, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Kim, S.J.; Kim, S.H.; Oh, C.D.; Hwang, S.G.; Chun, C.H.; Oh, S.H.; Seong, J.K.; Huh, T.L.; Chun, J.S. Regulation of the chondrocyte phenotype by beta-catenin. Development 2002, 129, 5541–5550. [Google Scholar] [CrossRef] [PubMed]
- Miclea, R.L.; Karperien, M.; Bosch, C.A.; van der Horst, G.; van der Valk, M.A.; Kobayashi, T.; Kronenberg, H.M.; Rawadi, G.; Akcakaya, P.; Lowik, C.W.; et al. Adenomatous polyposis coli-mediated control of beta-catenin is essential for both chondrogenic and osteogenic differentiation of skeletal precursors. BMC Dev. Biol. 2009, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattappa, G.; Heywood, H.K.; de Bruijn, J.D.; Lee, D.A. The metabolism of human mesenchymal stem cells during proliferation and differentiation. J. Cell. Physiol. 2011, 226, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, P.M.; van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Wang, Z.; Ruchalski, K.L.; Levine, J.S.; Krishnan, S.; Lieberthal, W.; Schwartz, J.H.; Borkan, S.C. Lithium activates the Wnt and phosphatidylinositol 3-kinase Akt signaling pathways to promote cell survival in the absence of soluble survival factors. Am. J. Physiol. Ren. Physiol. 2005, 288, F703–F713. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, M.; Zuscik, M.; Wu, Q.; Wang, Y.J.; Rosier, R.N.; O’Keefe, R.J.; Chen, D. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008, 58, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Tejado, M.; Naranjo-Suarez, S.; Jimenez, C.; Carrera, A.C.; Landazuri, M.O.; del Peso, L. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: Protective role in apoptosis. J. Biol. Chem. 2001, 276, 22368–22374. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Leijten, J.C.; Georgi, N.; Post, J.N.; van Blitterswijk, C.A.; Karperien, M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng. Part A 2011, 17, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Bonegio, R.; Lieberthal, W. Role of apoptosis in the pathogenesis of acute renal failure. Curr. Opin. Nephrol. Hypertens. 2002, 11, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.A.; Culbert, A.A.; Chalmers, K.A.; Facci, L.; Skaper, S.D.; Reith, A.D. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J. Neurochem. 2001, 77, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Lim, S.; Han, J.Y. Wnt/beta-catenin signaling pathway activation is required for proliferation of chicken primordial germ cells in vitro. Sci. Rep. 2016, 6, 34510. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Benoit, D.S. Agonism of Wnt-beta-catenin signalling promotes mesenchymal stem cell (MSC) expansion. J. Tissue Eng. Regen. Med. 2015, 9, E13–E26. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hou, Y.; Zhong, L.; Huang, D.; Qian, H.; Karperien, M.; Chen, W. Promoted Chondrogenesis of Cocultured Chondrocytes and Mesenchymal Stem Cells under Hypoxia Using In-situ Forming Degradable Hydrogel Scaffolds. Biomacromolecules 2018, 19, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Huang, X.; Karperien, M.; Post, J.N. Correlation between Gene Expression and Osteoarthritis Progression in Human. Int. J. Mol. Sci. 2016, 17, 1126. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Zhong, L.; Hendriks, J.; Post, J.N.; Karperien, M. The Effects of the WNT-Signaling Modulators BIO and PKF118-310 on the Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 561. https://doi.org/10.3390/ijms19020561
Huang X, Zhong L, Hendriks J, Post JN, Karperien M. The Effects of the WNT-Signaling Modulators BIO and PKF118-310 on the Chondrogenic Differentiation of Human Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2018; 19(2):561. https://doi.org/10.3390/ijms19020561
Chicago/Turabian StyleHuang, Xiaobin, Leilei Zhong, Jan Hendriks, Janine N. Post, and Marcel Karperien. 2018. "The Effects of the WNT-Signaling Modulators BIO and PKF118-310 on the Chondrogenic Differentiation of Human Mesenchymal Stem Cells" International Journal of Molecular Sciences 19, no. 2: 561. https://doi.org/10.3390/ijms19020561
APA StyleHuang, X., Zhong, L., Hendriks, J., Post, J. N., & Karperien, M. (2018). The Effects of the WNT-Signaling Modulators BIO and PKF118-310 on the Chondrogenic Differentiation of Human Mesenchymal Stem Cells. International Journal of Molecular Sciences, 19(2), 561. https://doi.org/10.3390/ijms19020561