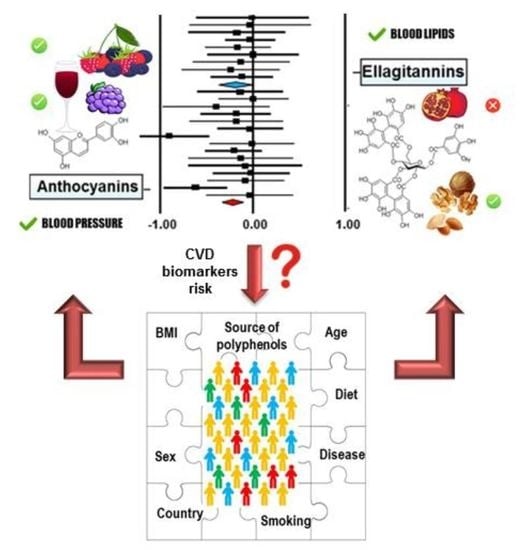
Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses
Abstract
:1. Introduction
2. Results
2.1. Description of the Selected Studies
2.2. Characteristics and Quality of the Included Studies
2.3. Overall Impact of Supplementation with Foods and Derived Products Containing ETs and/or ANCs on Biomarkers of Cardiovascular Risk
2.4. Comparative Analysis of the Potential Factors Influencing Interindividual Variability in the Responses to the Consumption of Foods and Food Products Containing ETs and ANCs
2.4.1. Stratification by the Individuals’ Baseline BMI, Sex, Smoking Habits and Background Diet
2.4.2. Stratification by the Health Status of the Participants
2.4.3. Stratification by the Country Where the Study Was Carried Out
2.4.4. Stratification by Specific Sources of ETs and ANCs.
3. Discussion
4. Materials and Methods
4.1. Search Strategy and Study Selection
4.2. Data Extraction
4.3. Assessment of the Risk of Bias
4.4. Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LDL-C | Low density lipoprotein cholesterol |
| HDL-C | High density lipoprotein cholesterol |
| ANCs | Anthocyanins |
| ETs | Ellagitannins |
| FW | Fresh weight |
| RCTs | Randomized-controlled trials |
| BMI | Body mass index |
| SDM | Standardized difference in means |
| DM | Difference in means |
| WC | Waist circumference |
| T-C | Total cholesterol |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| FMD | Flow mediated dilation |
| TAGs | Triglycerides |
| HbA1c | Glycated hemoglobin |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance |
| WHO | World health organization |
| NR | Not reported |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
References
- Papakonstantinou, E.; Lambadiari, V.; Dimitriadis, G.; Zampelas, A. Metabolic syndrome and cardiometabolic risk factors. Curr. Vasc. Pharmacol. 2013, 11, 858–879. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Riboli, E. Global health behavioral and dietary risk factors for non-communicable diseases. N. Engl. J. Med. 2013, 369, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Liu, Y.J.; Cai, L.B.; Xu, F.R.; Xie, T.; He, Q.Q. Fruit and vegetable consumption and risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Sofi, F. A heart-healthy diet: Recent insights and practical recommendations. Curr. Cardiol. Rep. 2017, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- Lecour, S.; Lamont, K.T. Natural polyphenols and cardioprotection. Mini-Rev. Med. Chem. 2011, 11, 1191–1199. [Google Scholar] [PubMed]
- Pinasseau, L.; Vallverdu-Queralt, A.; Verbaere, A.; Roques, M.; Meudec, E.; Le Cunff, L.; Peros, J.P.; Ageorges, A.; Sommerer, N.; Boulet, J.C.; et al. Cultivar diversity of grape skin polyphenol composition and changes in response to drought investigated by LC-MS based metabolomics. Front. Plant Sci. 2017, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Quaglieri, C.; Prieto-Perea, N.; Berrueta, L.A.; Gallo, B.; Rasines-Perea, Z.; Jourdes, M.; Teissedre, P.L. Comparison of Aquitaine and rioja red wines: Characterization of their phenolic composition and evolution from 2000 to 2013. Molecules 2017, 22, 192. [Google Scholar] [CrossRef] [PubMed]
- De Pascual-Teresa, S.; Moreno, D.A.; Garcia-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassidy, A. Berry anthocyanin intake and cardiovascular health. Mol. Asp. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-explorer 3.0: A major update of the phenol-explorer database to incorporate data on the effects of food processing on polyphenol content. Database J. Biol. Databases Curation 2013. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; O’Reilly, E.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.P.; Curhan, G.; Rimm, E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Bakkalbaşi, E.; Menteş, O.; Artik, N. Food ellagitannins-occurrence, effects of processing and storage. Crit. Rev. Food Sci. Nutr. 2009, 49, 283–298. [Google Scholar]
- Cerda, B.; Soto, C.; Albaladejo, M.D.; Martinez, P.; Sanchez-Gascon, F.; Tomas-Barberan, F.; Espin, J.C. Pomegranate juice supplementation in chronic obstructive pulmonary disease: A 5-week randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2006, 60, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Garcia-Conesa, M.T.; Espin, J.C.; Tomas-Barberan, F.A. Ellagitannins, ellagic acid and vascular health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.C.; Larrosa, M.; Garcia-Conesa, M.T.; Tomas-Barberan, F. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: The evidence so far. Evid.-Based Complement. Altern. Med. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.T.; Miao, Y.; Meng, Z.Y.; Zhong, Y. Effects of vaccinium berries on serum lipids: A meta-analysis of randomized controlled trials. Evid.-Based Complement. Altern. Med. 2015, 11. [Google Scholar] [CrossRef]
- Huang, H.H.; Chen, G.Z.; Liao, D.; Zhu, Y.K.; Xue, X.Y. Effects of berries consumption on cardiovascular risk factors: A meta-analysis with trial sequential analysis of randomized controlled trials. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Bo, Y.C.; Wang, X.; Lu, W.J.; Wang, X.L.; Han, Z.Y.; Qiu, C.G. The effect of anthocyanins on blood pressure a PRISMA-compliant meta-analysis of randomized clinical trials. Medicine 2016, 95, e3380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, J.; Lu, W.; Wang, X.; Han, Z.; Qiu, C. Effects of blueberry supplementation on blood pressure: A systematic review and meta-analysis of randomized clinical trials. J. Hum. Hypertens. 2017, 31, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.P.; Ling, W.H.; Du, Z.C.; Chen, Y.M.; Li, D.; Deng, S.Z.; Liu, Z.M.; Yang, L.L. Effects of anthocyanins on cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2017, 8, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The effect of anthocyanin-rich foods or extracts on vascular function in adults: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, N.; Salehi-Abargouei, A.; Salas-Salvado, J.; Guasch-Ferre, M.; Humphries, K.; Sarrafzadegan, N. The effect of tree nut, peanut, and soy nut consumption on blood pressure: A systematic review and meta-analysis of randomized controlled clinical trials. Am. J. Clin. Nutr. 2015, 101, 966–982. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, L.C.; Falk, M.C.; Feldman, R.; Lewis, K.; Mozaffarian, D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: Systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr. 2015, 102, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Ferri, C.; Giorgini, P.; Bo, S.; Nachtigal, P.; Grassi, D. Effects of pomegranate juice on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017, 115, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Simental-Mendia, L.E.; Giorgini, P.; Ferri, C.; Grassi, D. Lipid profile changes after pomegranate consumption: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine 2016, 23, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Haili, Z.; Shuang, L.; Lan, L.; Shisong, L.; Shuqi, L.; Jia, M.; Geng, T. The impact of grape seed extract treatment on blood pressure changes. Medicine (Baltimore) 2016, 95, e4247. [Google Scholar]
- Li, S.-H.; Zhao, P.; Tian, H.-B.; Chen, L.-H.; Cui, L.-Q. Effect of grape polyphenols on blood pressure: A meta-analysis of randomized controlled trials. PLoS ONE 2015, 10, e0137665. [Google Scholar]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrías, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.; Dumont, J.; et al. Impact of flavonols on cardiometabolic biomarkers: A meta-analysis of randomized controlled human trials to explore the role of inter-individual variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Combet, E.; Pinto, P.; Mena, P.; Dall’Asta, M.; Garcia-Aloy, M.; Rodríguez-Mateos, A.; Gibney, E.R.; Dumont, J.; Massaro, M.; et al. A systematic review and meta-analysis of the effects of flavanol-containing tea, cocoa and apple products on body composition and blood lipids: Exploring the factors responsible for variability in their efficacy. Nutrients 2017, 9, 746. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Del Pino-Garcia, R.; George, T.W.; Vidal-Diez, A.; Heiss, C.; Spencer, J.P.E. Impact of processing on the bioavailability and vascular effects of blueberry (poly)phenols. Mol. Nutr. Food Res. 2014, 58, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Orem, A.; Zafrilla, P.; Tomas-Barberan, F.A.; Selma, M.V.; Carlos Espin, J. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Kerimi, A.; Nyambe-Silavwe, H.; Gauer, J.S.; Tomás-Barberán, F.A.; Williamson, G. Pomegranate juice, but not an extract, confers a lower glycemic response on a high-glycemic index food: Randomized, crossover, controlled trials in healthy subjects. Am. J. Clin. Nutr. 2017, 106, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomas-Barberan, F.; et al. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Food Res. 2017, 61, 16. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amsha Caccetta, R.; Burke, V.; Mori, T.A.; Beilin, L.J.; Puddey, I.B.; Croft, K.D. Red wine polyphenols, in the absence of alcohol, reduce lipid peroxidative stress in smoking subjects. Free Radic. Biol. Med. 2001, 30, 636–642. [Google Scholar] [CrossRef]
- Asgary, S.; Kelishadi, R.; Rafieian-Kopaei, M.; Najafi, S.; Najafi, M.; Sahebkar, A. Investigation of the lipid-modifying and antiinflammatory effects of Cornus mas L. Supplementation on dyslipidemic children and adolescents. Pediatr. Cardiol. 2013, 34, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian-Kopaei, M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 2014, 28, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Banini, A.E.; Boyd, L.C.; Allen, J.C.; Allen, H.G.; Sauls, D.L. Muscadine grape products intake, diet and blood constituents of non-diabetic and type 2 diabetic subjects. Nutrition 2006, 22, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Betts, N.M.; Nguyen, A.; Newman, E.D.; Fu, D.; Lyons, T.J. Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J. Nutr. 2014, 144, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Betts, N.M.; Ortiz, J.; Simmons, B.; Wu, M.; Lyons, T.J. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr. Res. 2011, 31, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Fu, D.X.; Wilkinson, M.; Simmons, B.; Wu, M.; Betts, N.M.; Du, M.; Lyons, T.J. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr. Res. 2010, 30, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Bommannan Santhakumar, A.; Reddy Kundur, A.; Sabapathy, S.; Stanley, R.; Singh, I. The potential of anthocyanin-rich Queen Garnet plum juice supplementation in alleviating thrombotic risk under induced oxidative stress conditions. J. Funct. Foods 2015, 14, 747–757. [Google Scholar] [CrossRef]
- Botden, I.P.; Draijer, R.; Westerhof, B.E.; Rutten, J.H.; Langendonk, J.G.; Sijbrands, E.J.; Danser, A.H.; Zock, P.L.; van den Meiracker, A.H. Red wine polyphenols do not lower peripheral or central blood pressure in high normal blood pressure and hypertension. Am. J. Hypertens. 2012, 25, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.M.; Sweeney, L.L.; Liu, X.; Mantzoros, C.S. Walnut consumption increases satiation but has no effect on insulin resistance or the metabolic profile over a 4-day period. Obesity (Silver Spring) 2010, 18, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.; Linares, A.; Hyson, D.; Kappagoda, T. Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J. Am. Coll. Nutr. 2010, 29, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Caldu, P.; Hurtado, I.; Ramon, J.M.; Antoli, R.; Gonzalo, A.; Minguez, S.; Fiol, C. Changes in low density lipoprotein susceptibility to oxidation after wine ingestion. Medicina Clínica (Barcelona) 1998, 111, 451–455. [Google Scholar] [PubMed]
- Castilla, P.; Davalos, A.; Teruel, J.L.; Cerrato, F.; Fernandez-Lucas, M.; Merino, J.L.; Sanchez-Martin, C.C.; Ortuno, J.; Lasuncion, M.A. Comparative effects of dietary supplementation with red grape juice and vitamin e on production of superoxide by circulating neutrophil NADPH oxidase in hemodialysis patients. Am. J. Clin. Nutr. 2008, 87, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Castilla, P.; Echarri, R.; Davalos, A.; Cerrato, F.; Ortega, H.; Teruel, J.L.; Lucas, M.F.; Gomez-Coronado, D.; Ortuno, J.; Lasuncion, M.A. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am. J. Clin. Nutr. 2006, 84, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Arranz, S.; Valderas-Martinez, P.; Casas, R.; Sacanella, E.; Llorach, R.; Lamuela-Raventos, R.M.; Andres-Lacueva, C.; et al. Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide: Short communication. Circ. Res. 2012, 111, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P.M. Effect of grape seed extract and quercetin on cardiovascular and endothelial parameters in high-risk subjects. J. Biomed. Biotechnol. 2004, 2004, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.D.; Myers, S.D.; Blacker, S.D.; Willems, M.E. New Zealand blackcurrant extract improves cycling performance and fat oxidation in cyclists. Eur. J. Appl. Physiol. 2015, 115, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, A.M.; Guasch, V.; Castillo, O.; Irribarra, V.; Mizon, C.; San Martin, A.; Strobel, P.; Perez, D.; Germain, A.M.; Leighton, F. A high-fat diet induces and red wine counteracts endothelial dysfunction in human volunteers. Lipids 2000, 35, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Kroon, P.A.; Hollands, W.J.; Walls, R.; Jenkins, G.; Kay, C.D.; Cassidy, A. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J. Nutr. 2009, 139, 2266–2271. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Bertoglio, J.C.; Zarrelli, A.; Pina, R.; Scapagnini, G. A randomized clinical trial evaluating the efficacy of an anthocyanin-maqui berry extract (Delphinol®) on oxidative stress biomarkers. J. Am. Coll. Nutr. 2015, 34, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Porrini, M.; Fracassetti, D.; Campolo, J.; Klimis-Zacas, D.; Riso, P. A single serving of blueberry (V. corymbosum) modulates peripheral arterial dysfunction induced by acute cigarette smoking in young volunteers: A randomized-controlled trial. Food Funct. 2014, 5, 3107–3116. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Riso, P.; Campolo, J.; Moller, P.; Loft, S.; Klimis-Zacas, D.; Brambilla, A.; Rizzolo, A.; Porrini, M. A single portion of blueberry (Vaccinium corymbosum L) improves protection against DNA damage but not vascular function in healthy male volunteers. Nutr. Res. 2013, 33, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Din, J.N.; Aftab, S.M.; Jubb, A.W.; Carnegy, F.H.; Lyall, K.; Sarma, J.; Newby, D.E.; Flapan, A.D. Effect of moderate walnut consumption on lipid profile, arterial stiffness and platelet activation in humans. Eur. J. Clin. Nutr. 2011, 65, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Dohadwala, M.M.; Holbrook, M.; Hamburg, N.M.; Shenouda, S.M.; Chung, W.B.; Titas, M.; Kluge, M.A.; Wang, N.; Palmisano, J.; Milbury, P.E.; et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011, 93, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Draijer, R.; de Graaf, Y.; Slettenaar, M.; de Groot, E.; Wright, C.I. Consumption of a polyphenol-rich grape-wine extract lowers ambulatory blood pressure in mildly hypertensive subjects. Nutrients 2015, 7, 3138–3153. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Jenkinson, A.M.; Crozier, A.; Mullen, W.; Pirie, L.; Kyle, J.; Yap, L.S.; Christen, P.; Duthie, G.G. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.L.; Edirisinghe, I.; Kappagoda, T.; Burton-Freeman, B. Attenuation of meal-induced inflammatory and thrombotic responses in overweight men and women after 6-week daily strawberry (Fragaria) intake. A randomized placebo-controlled trial. J. Atheroscler. Thromb. 2011, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Koli, R.; Alfthan, G.; Marniemi, J.; Puukka, P.; Mustonen, P.; Mattila, P.; Jula, A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 2008, 87, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Sacanella, E.; Mota, F.; Chiva-Blanch, G.; Antunez, E.; Casals, E.; Deulofeu, R.; Rotilio, D.; Andres-Lacueva, C.; Lamuela-Raventos, R.M.; et al. Moderate consumption of red wine, but not gin, decreases erythrocyte superoxide dismutase activity: A randomised cross-over trial. Nutr. Metab. Cardiovas. Dis. 2011, 21, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Martin, E.A.; Gossl, M.; Widmer, R.J.; Lennon, R.J.; Sexton, J.A.; Loeffler, D.; Khosla, S.; Lerman, L.O.; Lerman, A. Polyphenol-rich cranberry juice has a neutral effect on endothelial function but decreases the fraction of osteocalcin-expressing endothelial progenitor cells. Eur. J. Nutr. 2013, 52, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.D.; Shantz, K.L.; Vander Veur, S.S.; Oliver, T.L.; Lent, M.R.; Virus, A.; Szapary, P.O.; Rader, D.J.; Zemel, B.S.; Gilden-Tsai, A. A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. Am. J. Clin. Nutr. 2012, 96, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Lavy, A.; Aviram, M. Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation. Am. J. Clin. Nutr. 1995, 61, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.M.; Garovic-Kocic, V.; Diamandis, E.P.; Pace-Asciak, C.R. Wine: Does the color count? Clin. Chim. Acta 1996, 246, 183–193. [Google Scholar] [CrossRef]
- Gorinstein, S.; Caspi, A.; Libman, I.; Lerner, H.T.; Huang, D.; Leontowicz, H.; Leontowicz, M.; Tashma, Z.; Katrich, E.; Feng, S.; et al. Red grapefruit positively influences serum triglyceride level in patients suffering from coronary atherosclerosis: Studies in vitro and in humans. J. Agric. Food Chem. 2006, 54, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Misra, A.; Pandey, R.M.; Bhatt, S.P.; Saluja, S. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: A 24-wk, randomized control trial. Nutrition 2014, 30, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhong, R.; Liu, Y.; Jiang, X.; Tang, X.; Li, Z.; Xia, M.; Ling, W. Effects of bayberry juice on inflammatory and apoptotic markers in young adults with features of non-alcoholic fatty liver disease. Nutrition 2014, 30, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.H.; Gaban-Chong, N.; Oda, K.; Sabaté, J. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr. J. 2014, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.S.; Marckmann, P.; Dragsted, L.O.; Finne Nielsen, I.L.; Nielsen, S.E.; Gronbaek, M. Effect of red wine and red grape extract on blood lipids, hemostatic factors, and other risk factors for cardiovascular disease. Eur. J. Clin. Nutr. 2005, 59, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Hassellund, S.S.; Flaa, A.; Kjeldsen, S.E.; Seljeflot, I.; Karlsen, A.; Erlund, I.; Rostrup, M. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: A double-blind randomized placebo-controlled crossover study. J. Hum. Hypertens. 2013, 27, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Seeram, N.P.; Wyatt, H.; Henning, S.M.; Zhang, Y.; Ogden, L.G.; Dreher, M.; Hill, J.O. Safety and antioxidant activity of a pomegranate ellagitannin-enriched polyphenol dietary supplement in overweight individuals with increased waist size. J. Agric. Food Chem. 2007, 55, 10050–10054. [Google Scholar] [CrossRef] [PubMed]
- Hijmering, M.L.; de Lange, D.W.; Lorsheyd, A.; Kraaijenhagen, R.J.; van de Wiel, A. Binge drinking causes endothelial dysfunction, which is not prevented by wine polyphenols: A small trial in healthy volunteers. Neth. J. Med. 2007, 65, 29–35. [Google Scholar] [PubMed]
- Hoggard, N.; Cruickshank, M.; Moar, K.M.; Bestwick, C.; Holst, J.J.; Russell, W.; Horgan, G. A single supplement of a standardised bilberry (Vaccinium myrtillus L.) extract (36% wet weight anthocyanins) modifies glycemic response in individuals with type 2 diabetes controlled by diet and lifestyle. J. Nutr. Sci. 2013, 2, e22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hokayem, M.; Blond, E.; Vidal, H.; Lambert, K.; Meugnier, E.; Feillet-Coudray, C.; Coudray, C.; Pesenti, S.; Luyton, C.; Lambert-Porcheron, S.; et al. Grape polyphenols prevent fructose-induced oxidative stress and insulin resistance in first-degree relatives of type 2 diabetic patients. Diabetes Care 2013, 36, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Hollis, J.H.; Houchins, J.A.; Blumberg, J.B.; Mattes, R.D. Effects of concord grape juice on appetite, diet, body weight, lipid profile, and antioxidant status of adults. J. Am. Coll. Nutr. 2009, 28, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Saedisomeolia, A.; Wood, L.G.; Yaseri, M.; Tavasoli, S. Effects of pomegranate extract supplementation on inflammation in overweight and obese individuals: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2016, 22, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Huebbe, P.; Giller, K.; de Pascual-Teresa, S.; Arkenau, A.; Adolphi, B.; Portius, S.; Arkenau, C.N.; Rimbach, G. Effects of blackcurrant-based juice on atherosclerosis-related biomarkers in cultured macrophages and in human subjects after consumption of a high-energy meal. Br. J. Nutr. 2012, 108, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Nguyen, T.H.; Kendall, C.W.; Faulkner, D.A.; Bashyam, B.; Kim, I.J.; Ireland, C.; Patel, D.; Vidgen, E.; Josse, A.R.; et al. The effect of strawberries in a cholesterol-lowering dietary portfolio. Metabolism 2008, 57, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.P.; Serrano, J.; Tabernero, M.; Arranz, S.; Diaz-Rubio, M.E.; Garcia-Diz, L.; Goni, I.; Saura-Calixto, F. Effects of grape antioxidant dietary fiber in cardiovascular disease risk factors. Nutrition 2008, 24, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Alimbetov, D.; George, T.; Gordon, M.H.; Lovegrove, J.A. A randomized trial to investigate the effects of acute consumption of a blackcurrant juice drink on markers of vascular reactivity and bioavailability of anthocyanins in human subjects. Eur. J. Nutr. 2011, 65, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E.; et al. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: A randomized, double-blind, placebo-controlled clinical trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Trier, C.M.; Fleming, K.R. The effect of peanut and grain bar preloads on post-meal satiety, glycaemia, and weight loss in healthy individuals: An acute and a chronic randomized intervention trial. Nutr. J. 2013, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Karatzi, K.; Papamichael, C.; Aznaouridis, K.; Karatzis, E.; Lekakis, J.; Matsouka, C.; Boskou, G.; Chiou, A.; Sitara, M.; Feliou, G.; et al. Constituents of red wine other than alcohol improve endothelial function in patients with coronary artery disease. Coron. Artery Dis. 2004, 15, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Paur, I.; Bøhn, S.K.; Sakhi, A.K.; Borge, G.I.; Serafini, M.; Erlund, I.; Laake, P.; Tonstad, S.; Blomhoff, R. Bilberry juice modulates plasma concentration of NF-κB related inflammatory markers in subjects at increased risk of CVD. Eur. J. Nutr. 2010, 49, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.L.; Davidhi, A.; Ma, Y.; Kavak, Y.; Bifulco, L.; Njike, V.Y. Effects of walnuts on endothelial function in overweight adults with visceral obesity: A randomized, controlled, crossover trial. J. Am. Coll. Nutr. 2012, 31, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Holub, B.J. The effect of wild blueberry (Vaccinium angustifolium) consumption on postprandial serum antioxidant status in human subjects. Br. J. Nutr. 2002, 88, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Ray, S.; Craigie, A.M.; Kennedy, G.; Hill, A.; Barton, K.L.; Broughton, J.; Belch, J.J. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: A randomized controlled trial of antioxidant- and polyphenol-rich blackcurrant juice. Free Radic. Biol. Med. 2014, 72, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kianbakht, S.; Abasi, B.; Dabaghian, F.H. Anti-hyperglycemic effect of Vaccinium arctostaphylos in type 2 diabetic patients: A randomized controlled trial. Forsch. Komplement. 2013, 20, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, M.; Mykkanen, O.; Kirjavainen, P.V.; Leppanen, T.; Moilanen, E.; Adriaens, M.; Laaksonen, D.E.; Hallikainen, M.; Puupponen-Pimia, R.; Pulkkinen, L.; et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012, 56, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Boespflug, E.L.; Fleck, D.E.; Stein, A.L.; Wightman, J.D.; Shidler, M.D.; Sadat-Hossieny, S. Concord grape juice supplementation and neurocognitive function in human aging. J. Agric. Food Chem. 2012, 60, 5736–5742. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.J.; Lawton, C.L.; Merat, N.; Jamson, H.; Myrissa, K.; Hofman, D.; Chadwick, H.K.; Quadt, F.; Wightman, J.D.; Dye, L. Concord grape juice, cognitive function, and driving performance: A 12-wk, placebo-controlled, randomized crossover trial in mothers of preteen children. Am. J. Clin. Nutr. 2016, 103, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Nam, G.E.; Seo, J.A.; Yoon, T.; Seo, I.; Lee, J.H.; Im, D.; Bahn, K.N.; Jeong, S.A.; Kang, T.S.; et al. Nut consumption has favorable effects on lipid profiles of Korean women with metabolic syndrome. Nutr. Res. 2014, 34, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Lekakis, J.; Rallidis, L.S.; Andreadou, I.; Vamvakou, G.; Kazantzoglou, G.; Magiatis, P.; Skaltsounis, A.L.; Kremastinos, D.T. Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Eur. J. Cardiovasc. Prev. Rehabilit. 2005, 12, 596–600. [Google Scholar] [CrossRef]
- Li, Z.; Song, R.; Nguyen, C.; Zerlin, A.; Karp, H.; Naowamondhol, K.; Thames, G.; Gao, K.; Li, L.; Tseng, C.H.; et al. Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J. Am. Coll. Nutr. 2010, 29, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Uriarte, P.; Nogues, R.; Saez, G.; Bullo, M.; Romeu, M.; Masana, L.; Tormos, C.; Casas-Agustench, P.; Salas-Salvado, J. Effect of nut consumption on oxidative stress and the endothelial function in metabolic syndrome. Clin. Nutr. 2010, 29, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Hamadeh, H.; Leung, W.C.; Russell, J.M.; Barker, M.E. Effects of pomegranate juice supplementation on pulse wave velocity and blood pressure in healthy young and middle-aged men and women. Plant Foods Hum. Nutr. 2012, 67, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Mathew, S.; Moore, C.T.; Russell, J.; Robinson, E.; Soumpasi, V.; Barker, M.E. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: A randomized controlled trial. Plant Foods Hum. Nutr. 2014, 69, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Njike, V.Y.; Millet, J.; Dutta, S.; Doughty, K.; Treu, J.A.; Katz, D.L. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: A randomized controlled crossover trial. Diabetes Care 2010, 33, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.S.; Capel-Williams, G.M.; Berry, S.E.E.; Hall, W.L. Acute effects of pomegranate extract on postprandial lipemia, vascular function and blood pressure. Plant Foods Hum. Nutr. 2012, 67, 351–357. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, L.S.; Collier, S.R.; Landram, M.J.; Whittaker, D.S.; Isaacs, S.E.; Klemka, J.M.; Cheek, S.L.; Arms, J.C.; McAnulty, S.R. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr. Res. 2014, 34, 577–584. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, S.R.; McAnulty, L.S.; Morrow, J.D.; Khardouni, D.; Shooter, L.; Monk, J.; Gross, S.; Brown, V. Effect of daily fruit ingestion on angiotensin converting enzyme activity, blood pressure, and oxidative stress in chronic smokers. Free Radic. Res. 2005, 39, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Chen, C.Y.; Saltzman, E.; Blumberg, J.B. Hibiscus sabdariffa l. Tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J. Nutr. 2010, 140, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Moazen, S.; Amani, R.; Homayouni Rad, A.; Shahbazian, H.; Ahmadi, K.; Taha Jalali, M. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: A randomized double-blind controlled trial. Ann. Nutr. Metab. 2013, 63, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Mukuddem-Petersen, J.; Stonehouse Oosthuizen, W.; Jerling, J.C.; Hanekom, S.M.; White, Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: A controlled feeding trial. Br. J. Nutr. 2007, 97, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Naissides, M.; Mamo, J.C.; James, A.P.; Pal, S. The effect of acute red wine polyphenol consumption on postprandial lipemia in postmenopausal women. Atherosclerosis 2004, 177, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Naissides, M.; Mamo, J.C.; James, A.P.; Pal, S. The effect of chronic consumption of red wine on cardiovascular disease risk factors in postmenopausal women. Atherosclerosis 2006, 185, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Macone, A.; Ramberti, A.; Forte, M.; Mattivi, F.; Matarese, R.M.; Scaccini, C. Red wine prevents the postprandial increase in plasma cholesterol oxidation products: A pilot study. Br. J. Nutr. 2011, 105, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Nigdikar, S.V.; Williams, N.R.; Griffin, B.A.; Howard, A.N. Consumption of red wine polyphenols reduces the susceptibility of low-density lipoproteins to oxidation in vivo. Am. J. Clin. Nutr. 1998, 68, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Novotny, J.A.; Baer, D.J.; Khoo, C.; Gebauer, S.K.; Charron, C.S. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating c-reactive protein, triglyceride, and glucose concentrations in adults. J. Nutr. 2015, 145, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Ohguro, H.; Ohguro, I.; Katai, M.; Tanaka, S. Two-year randomized, placebo-controlled study of black currant anthocyanins on visual field in glaucoma. Ophthalmologica 2012, 228, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla-Alonso, B.; Granado-Lorencio, F.; Herrero-Barbudo, C.; Blanco-Navarro, I.; Blazquez-Garcia, S.; Perez-Sacristan, B. Consumption of restructured meat products with added walnuts has a cholesterol-lowering effect in subjects at high cardiovascular risk: A randomized, crossover, placebo-controlled study. J. Am. Coll. Nutr. 2008, 27, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Parham, M.; Heidari, S.; Khorramirad, A.; Hozoori, M.; Hosseinzadeh, F.; Bakhtyari, L.; Vafaeimanesh, J. Effects of pistachio nut supplementation on blood glucose in patients with type 2 diabetes: A randomized crossover trial. Rev. Diabet. Stud. 2014, 11, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, J.Y.; Kim, J.; Kim, Y.J.; Kim, M.J.; Kwon, S.W.; Kwon, O. Pomegranate vinegar beverage reduces visceral fat accumulation in association with AMPK activation in overweight women: A double-blind, randomized, and placebo-controlled trial. J. Funct. Foods 2014, 8, 274–281. [Google Scholar] [CrossRef]
- Park, Y.K.; Kim, J.S.; Kang, M.H. Concord grape juice supplementation reduces blood pressure in Korean hypertensive men: Double-blind, placebo controlled intervention trial. BioFactors 2004, 22, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Wallerstedt, D.; Talpur, N.; Tutuncuoglu, S.O.; Echard, B.; Myers, A.; Bui, M.; Bagchi, D. Effects of niacin-bound chromium and grape seed proanthocyanidin extract on the lipid profile of hypercholesterolemic subjects: A pilot study. J. Med. 2000, 31, 227–246. [Google Scholar] [PubMed]
- Puupponen-Pimiä, R.; Seppänen-Laakso, T.; Kankainen, M.; Maukonen, J.; Törrönen, R.; Kolehmainen, M.; Leppänen, T.; Moilanen, E.; Nohynek, L.; Aura, A.-M.; et al. Effects of ellagitannin-rich berries on blood lipids, gut microbiota, and urolithin production in human subjects with symptoms of metabolic syndrome. Mol. Nutr. Food Res. 2013, 57, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J.; Mou, H.; Cao, L.; Ling, W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.R.; Mahmoudabadi, M.M.; Islam, M.S. Comparative effects of red and white grapes on oxidative markers and lipidemic parameters in adult hypercholesterolemic humans. Food Funct. 2015, 6, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Zock, P.L.; Zebregs, Y.E.; Johnston, N.R.; Webb, D.J.; Draijer, R. Effect of polyphenol-rich grape seed extract on ambulatory blood pressure in subjects with pre- and stage I hypertension. Br. J. Nutr. 2013, 110, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Gholamin, S.; Eskandari, A.; Mohsenian, N.; Ghorbanihaghjo, A.; Delazar, A.; Rashtchizadeh, N.; Keshtkar-Jahromi, M.; Argani, H. Red grape seed extract improves lipid profiles and decreases oxidized low-density lipoprotein in patients with mild hyperlipidemia. J. Med. Food 2013, 16, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.; Klimis-Zacas, D.; Del Bo, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P.E. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Ruel, G.; Lapointe, A.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Evidence that cranberry juice may improve augmentation index in overweight men. Nutr. Res. 2013, 33, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Shema-Didi, L.; Kristal, B.; Sela, S.; Geron, R.; Ore, L. Does pomegranate intake attenuate cardiovascular risk factors in hemodialysis patients? Nutr. J. 2014, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasapillai, B.; Edirisinghe, I.; Randolph, J.; Steinberg, F.; Kappagoda, T. Effect of grape seed extract on blood pressure in subjects with the metabolic syndrome. Metabolism 2009, 58, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Spaak, J.; Merlocco, A.C.; Soleas, G.J.; Tomlinson, G.; Morris, B.L.; Picton, P.; Notarius, C.F.; Chan, C.T.; Floras, J.S. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H605–H612. [Google Scholar] [CrossRef] [PubMed]
- Spaccarotella, K.J.; Kris-Etherton, P.M.; Stone, W.L.; Bagshaw, D.M.; Fishell, V.K.; West, S.G.; Lawrence, F.R.; Hartman, T.J. The effect of walnut intake on factors related to prostate and vascular health in older men. Nutr. J. 2008, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Sumner, M.D.; Elliott-Eller, M.; Weidner, G.; Daubenmier, J.J.; Chew, M.H.; Marlin, R.; Raisin, C.J.; Ornish, D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am. J. Cardiol. 2005, 96, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: A randomized, double-blind, placebo-controlled pilot study. Menopause 2014, 21, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.L.; Gray, R.A.; Chisholm, W.A.; Delahunty, M.C.; Brown, C.R. The dose of hazelnuts influences acceptance and diet quality but not inflammatory markers and body composition in overweight and obese individuals. J. Nutr. 2013, 143, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Garcia-Almagro, F.J.; Aviles-Plaza, F.; Parra, S.; Yanez-Gascon, M.J.; Ruiz-Ros, J.A.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and APOB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Larrosa, M.; Yanez-Gascon, M.J.; Davalos, A.; Gil-Zamorano, J.; Gonzalvez, M.; Garcia-Almagro, F.J.; Ruiz Ros, J.A.; Tomas-Barberan, F.A.; Espin, J.C.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.; Higgins, S.; Duthie, G.G.; Duthie, S.J.; Howie, M.; Mullen, W.; Lean, M.E.; Crozier, A. The influence of moderate red wine consumption on antioxidant status and indices of oxidative stress associated with CHD in healthy volunteers. Br. J. Nutr. 2005, 93, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.; Smail, N.F.; Almoosawi, S.; Davidson, I.; Al-Dujaili, E.A. Intake of polyphenol-rich pomegranate pure juice influences urinary glucocorticoids, blood pressure and homeostasis model assessment of insulin resistance in human volunteers. J. Nutr. Sci. 2012, 1, e9. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Depont, F.; Jacquet, A.; Lignot, S.; Segur, M.C.; Boisseau, M.; Begaud, B.; Moore, N. Effects of Armagnac or vodka on platelet aggregation in healthy volunteers: A randomized controlled clinical trial. Thromb. Res. 2005, 115, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; D’Acuna, S.; Perez, D.; Dicenta, S.; Echeverria, G.; Rigotti, A.; Leighton, F. Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: A randomized controlled trial. Biol. Res. 2015, 48, 49. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, N.; Niv, E. Daily consumption of red grape cell powder in a dietary dose improves cardiovascular parameters: A double blind, placebo-controlled, randomized study. Int. J. Food Sci. Nutr. 2015, 66, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Velliquette, R.A.; Grann, K.; Missler, S.R.; Patterson, J.; Hu, C.; Gellenbeck, K.W.; Scholten, J.D.; Randolph, R.K. Identification of a botanical inhibitor of intestinal diacylglyceride acyltransferase 1 activity via in vitro screening and a parallel, randomized, blinded, placebo-controlled clinical trial. Nutr. Metab. 2015, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Vidlar, A.; Vostalova, J.; Ulrichova, J.; Student, V.; Stejskal, D.; Reichenbach, R.; Vrbkova, J.; Ruzicka, F.; Simanek, V. The effectiveness of dried cranberries (Vaccinium macrocarpon) in men with lower urinary tract symptoms. Br. J. Nutr. 2010, 104, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Liu, Y.; Lv, X.; Yang, W. Effects of pistachios on body weight in Chinese subjects with metabolic syndrome. Nutr. J. 2012, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.W.; Haskell-Ramsay, C.F.; Kennedy, D.O.; Cooney, J.M.; Trower, T.; Scheepens, A. Acute supplementation with blackcurrant extracts modulates cognitive functioning and inhibits monoamine oxidase-B in healthy young adults. J. Funct. Foods 2015, 17, 524–539. [Google Scholar] [CrossRef]
- Whelan, A.P.; Sutherland, W.H.; McCormick, M.P.; Yeoman, D.J.; de Jong, S.A.; Williams, M.J. Effects of white and red wine on endothelial function in subjects with coronary artery disease. Intern. Med. J. 2004, 34, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.; Bleich, D.; Raghuwanshi, M.; Gould-Forgerite, S.; Gomes, J.; Monahan-Couch, L.; Oda, K. Almond consumption and cardiovascular risk factors in adults with prediabetes. J. Am. Coll. Nutr. 2010, 29, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.J.; Sutherland, W.H.; Whelan, A.P.; McCormick, M.P.; de Jong, S.A. Acute effect of drinking red and white wines on circulating levels of inflammation-sensitive molecules in men with coronary artery disease. Metabolism 2004, 53, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Piotrowski, K.; Rau, T.; Waldmann, E.; Broedl, U.C.; Demmelmair, H.; Koletzko, B.; Stark, R.G.; Nagel, J.M.; Mantzoros, C.S.; et al. Walnut-enriched diet reduces fasting non-HDL-cholesterol and Apolipoprotein B in healthy Caucasian subjects: A randomized controlled cross-over clinical trial. Metabolism 2014, 63, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.T.; Fitschen, P.J.; Kistler, B.M.; Jeong, J.H.; Chung, H.R.; Aviram, M.; Phillips, S.A.; Fernhall, B.; Wilund, K.R. Effects of pomegranate extract supplementation on cardiovascular risk factors and physical function in hemodialysis patients. J. Med. Food 2015, 18, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Yubero, N.; Sanz-Buenhombre, M.; Guadarrama, A.; Villanueva, S.; Carrion, J.M.; Larrarte, E.; Moro, C. LDL cholesterol-lowering effects of grape extract used as a dietary supplement on healthy volunteers. Int. J. Food Sci. Nutr. 2013, 64, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Zern, T.L.; Wood, R.J.; Greene, C.; West, K.L.; Liu, Y.; Aggarwal, D.; Shachter, N.S.; Fernandez, M.L. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J. Nutr. 2005, 135, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.W.; Chen, F.X.; Li, D.; Ling, W.H.; Guo, H.H. A consort-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine (Baltimore) 2015, 94, e758. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xia, M.; Yang, Y.; Liu, F.; Li, Z.; Hao, Y.; Mi, M.; Jin, T.; Ling, W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin. Chem. 2011, 57, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Zunino, S.J.; Peerson, J.M.; Freytag, T.L.; Breksa, A.P.; Bonnel, E.L.; Woodhouse, L.R.; Storms, D.H. Dietary grape powder increases IL-1β and Il-6 production by lipopolysaccharide-activated monocytes and reduces plasma concentrations of large LDL and large LDL-cholesterol particles in obese humans. Br. J. Nutr. 2014, 112, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Torronen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Kahkonen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R.; Prospective Studies, C. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [PubMed]
- Ginsberg, H. Statins in cardiometabolic disease: What makes pitavastatin different? Cardiovasc. Diabetol. 2013, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, C.; Parati, G.; Zanchetti, A. Effects of blood pressure lowering on outcome incidence in hypertension: 4. Effects of various classes of antihypertensive drugs-overview and meta-analyses. J. Hypertens. 2015, 33, 195–211. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Waist Circumference and Waist–Hip Ratio. In Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2011; pp. 1–39. ISBN 978-92-4-150149-1. [Google Scholar]
- Sindone, A.; Erlich, J.; Lee, C.; Newman, H.; Suranyi, M.; Roger, S.D. Cardiovascular risk reduction in hypertension: Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers. Where are we up to? Intern. Med. J. 2016, 46, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Jimmie Leppink, J.; O’Sullivan, P.; Winston, K. Effect size—Large, medium, and small. Perspect. Med. Educ. 2016, 5, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C. Current and future directions of cardiovascular risk prediction. Am. J. Cardiol. 2006, 97, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Basile, J.; Houston, M.; Ferrario, C.M. Treating the cardiometabolic syndrome: An opportunity to provide comprehensive cardiovascular risk reduction. J. Cardiometab. Syndr. 2006, 1, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Slavin, M.; Frankenfeld, C.L. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients 2016, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Sun, J.F.; Lu, Y.; Bo, Y.C. Effects of anthocyanin on serum lipids in dyslipidemia patients: A systematic review and meta-analysis. PLoS ONE 2016, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Chiu, A.; Barone, M.K.; Avino, D.; Wang, F.; Coleman, C.I.; Phung, O.J. Green tea catechins decrease total and low-density lipoprotein cholesterol: A systematic review and meta-analysis. J. Am. Dietetic Assoc. 2011, 111, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.X.; Xu, Y.L.; Li, S.H.; Liu, X.X.; Hui, R.T.; Huang, X.H. Green tea intake lowers fasting serum total and LDL cholesterol in adults: A meta-analysis of 14 randomized controlled trials. Am. J. Clin. Nutr. 2011, 94, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Asimi, S.; Wu, K.J.; Zheng, J.S.; Li, D. Black tea consumption and serum cholesterol concentration: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2015, 34, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, X.A.; Bai, Y.Y.; Li, S.H.; Sun, K.; He, C.; Hui, R.T. Short-term effect of cocoa product consumption on lipid profile: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2010, 92, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Tokede, O.A.; Onabanjo, T.A.; Yansane, A.; Gaziano, J.M.; Djousse, L. Soya products and serum lipids: A meta-analysis of randomized controlled trials. Br. J. Nutr. 2015, 114, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G.; Missbach, B.; Stelmach-Mardas, M.; Boeing, H. An umbrella review of nuts intake and risk of cardiovascular disease. Curr. Pharm. Des. 2017, 23, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, A.J.; de Souza, R.J.; Meyre, D.; Anand, S.S.; Mente, A. A systematic review and meta-analysis of nut consumption and incident risk of CVD and all-cause mortality. Br. J. Nutr. 2016, 115, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.Z.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A C-13-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.J.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Selma, M.V.; Espin, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Van de Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sarrias, A.; Espin, J.C.; Tomas-Barberan, F.A. Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects. Trends Food Sci. Technol. 2017, 69, 281–288. [Google Scholar] [CrossRef]
- Frankenfeld, C.L.; Atkinson, C.; Wahala, K.; Lampe, J.W. Obesity prevalence in relation to gut microbial environments capable of producing equol or o-desmethylangolensin from the isoflavone daidzein. Eur. J. Clin. Nutr. 2014, 68, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Newton, K.M.; Yong, M.; Stanczyk, F.Z.; Westerlind, K.C.; Li, L.; Lampe, J.W. Daidzein-metabolizing phenotypes in relation to bone density and body composition among premenopausal women in the united states. Metab.-Clin. Exp. 2012, 61, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Conesa, M.T. Dietary polyphenols against metabolic disorders: How far have we progressed in the understanding of the molecular mechanisms of action of these compounds? Crit. Rev. Food Sci. Nutr. 2017, 57, 1769–1786. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Ordovas, J.M. Can genotype be used to tailor treatment of obesity? State of the art and guidelines for future studies and applications. Minerva Endocrinol. 2013, 38, 219–235. [Google Scholar] [PubMed]
- Miller, R.J.; Jackson, K.G.; Dadd, T.; Mayes, A.E.; Brown, A.L.; Lovegrove, J.A.; Minihane, A.M. The impact of the catechol-O-methyltransferase genotype on vascular function and blood pressure after acute green tea ingestion. Mol. Nutr. Food Res. 2012, 56, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Wakeling, L.A.; Ford, D. Polymorphisms in genes involved in the metabolism and transport of soy isoflavones affect the urinary metabolite profile in premenopausal women following consumption of a commercial soy supplement as a single bolus dose. Mol. Nutr. Food Res. 2012, 56, 1794–1802. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Boesch-Saadatmandi, C.; Wolffram, S.; Rimbach, G.; Muller, M.J. Serum lipid and blood pressure responses to quercetin vary in overweight patients by apolipoprotein e genotype. J. Nutr. 2010, 140, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.W.; Koch, T.C.L.; Watzl, B.; Dietrich, H.; Will, F.; Bub, A. Moderate effects of apple juice consumption on obesity-related markers in obese men: Impact of diet-gene interaction on body fat content. Eur. J. Nutr. 2012, 51, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Flather, M.D.; Farkouh, M.E.; Pogue, J.M.; Yusuf, S. Strengths and limitations of meta-analysis: Larger studies may be more reliable. Control. Clin. Trials 1997, 18, 568–579. [Google Scholar] [CrossRef]
- Cumming, G. The new statistics: Why and how. Physiol. Sci. 2014, 25, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]; The Cochrane Collaboration: London, UK, 2011; Available online: www.handbook.cochrane.org (accessed on 1 April 2016).
- Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care; Centre for Reviews and Dissemination: Heslington, UK, 2008. [Google Scholar]
- PROSPERO. Available online: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016037539 (accessed on 9 April 2016).
- MEDLINE. Available online: http://www.ncbi.nlm.nih.gov/pubmed (accessed on 10 April 2016).
- Web of Knowledge. Available online: http://apps.webofknowledge.com (accessed on 10 April 2016).
- Borenstein, M.J.; Hedges, L.V.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis, version 3.3; Biostat, Inc.: Englewood, NJ, USA, 2014. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedgwick, P.; Marston, L. How to read a funnel plot in a meta-analysis. BMJ 2015, 351, h4718. [Google Scholar] [CrossRef] [PubMed]

| SDM (p-Value) | 95% CI | n | NT | I2 (%) | GRADE 1 | DM (p-Value) | 95% CI | n | NT | I2 (%) | GRADE | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WC | −0.30 (0.008) | (−0.52, −0.08) | 23 | 1023 | 63.4 | Moderate 2 | WC (cm) | −1.22 (0.005) | (−2.07, −0.36) | 22 | 972 | 37.5 | Moderate 2 |
| T-C | −0.17 (0.001) | (−0.27, −0.07) | 109 | 3991 | 54.5 | Moderate 2 | T-C (mmol/L) | −0.10 (0.013) | (−0.18, −0.02) | 103 | 3673 | 70.6 | Moderate 2 |
| SBP | −0.20 (0.000) | (−0.28, −0.12) | 95 | 3539 | 25.0 | High | SBP (mm Hg) | −1.56 (0.000) | (−2.13, −0.99) | 83 | 3175 | 0.00 | High |
| DBP | −0.19 (0.000) | (−0.26, −0.11) | 99 | 3790 | 27.9 | High | DBP (mm Hg) | −1.42 (0.000) | (−2.08, −0.76) | 90 | 3473 | 41.6 | Moderate 2 |
| HDL-C | +0.11 (0.034) | (0.01, 0.21) | 99 | 3581 | 53.1 | Low 2,3 | HDL-C (mmol/L) | +0.03 (0.062) | (0.00, 0.05) | 92 | 3239 | 61.6 | Moderate 2 |
| FMD | +0.20 (NS) | (−0.17, 0.57) | 22 | 563 | 73.8 | Low 2,3 | FMD (%) | +0.64 (0.027) | (0.07, 1.20) | 21 | 547 | 82.2 | Low 2,3 |
| ET-Containing Products (Pomegranate, Nuts) | ANC-Containing Products (Berries, Red Grapes, Red Wine) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SDM (p-Value) | n | DM (p-Value) | n | SDM (p-Value) | n | DM (p-Value) | n | ||||
| WC | −0.70 (0.025) | 7 | WC (cm) | −1.53 (0.031) | 6 | WC | −0.12 (NS) | 16 | WC (cm) | −0.75 (NS) | 16 |
| T-C | −0.18 (0.006) | 28 | T-C (mmol/L) | −0.09 (0.000) | 26 | T-C | −0.17 (0.008) | 81 | T-C (mmol/L) | −0.10 (0.094) | 77 |
| HDL-C | +0.10 (NS) | 23 | HDL-C (mmol/L) | +0.03 (NS) | 21 | HDL-C | +0.12 (NS) | 76 | HDL-C (mmol/L) | +0.03 (NS) | 71 |
| LDL-C | −0.19 (0.031) | 26 | LDL-C (mmol/L) | −0.11 (0.000) | 24 | LDL-C | 0.05 (NS) | 71 | LDL-C (mmol/L) | −0.03 (NS) | 68 |
| TAGs | −0.24 (0.025) | 26 | TAGS (mmol/L) | −0.11 (0.000) | 24 | TAGs | +0.004 (NS) | 71 | TAGS (mmol/L) | +0.02 (NS) | 64 |
| SBP | −0.11 (NS) | 21 | SBP (mm Hg) | −1.89 (NS) | 15 | SBP | −0.23 (0.000) | 74 | SBP (mm Hg) | −2.19 (0.000) | 68 |
| DBP | −0.14 (NS) | 20 | DBP (mm Hg) | −1.28 (NS) | 18 | DBP | −0.20 (0.000) | 79 | DBP (mm Hg) | −1.58 (0.000) | 72 |
| FMD | +0.62 (0.014) | 3 | FMD (%) | +0.39 (NS) | 3 | FMD | +0.12 (NS) | 19 | FMD (%) | +0.53 (NS) | 18 |
| Glucose | −0.24 (0.052) | 16 | Glucose (mmol/L) | −0.12 (0.01) | 15 | Glucose | −0.05 (NS) | 22 | Glucose (mmol/L) | +0.001 (NS) | 45 |
| BMI ≥ 25.0 kg/m2 | ET-Containing Products (Pomegranate, Nuts) | ANC-Containing Products (Berries, Red Grapes, Red Wine) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SDM (p-Value) | n | DM (p-Value) | n | SDM (p-Value) | n | DM (p-Value) | n | ||||
| WC | −0.70 (NS) | 4 | WC (cm) | −1.71 (0.047) | 3 | WC | −0.11 (NS) | 10 | WC (cm) | −0.70 (NS) | 10 |
| T-C | −0.12 (NS) | 20 | T-C (mmol/L) | −0.08 (0.000) | 18 | T-C | −0.34 (0.003) | 35 | T-C (mmol/L) | −0.23 (0.009) | 35 |
| LDL-C | −0.15 (NS) | 20 | LDL-C (mmol/L) | −0.11, (0.000) | 18 | LDL-C | −0.13 (NS) | 30 | LDL-C (mmol/L) | −0.11 (NS) | 29 |
| TAGs | −0.21 (NS) | 19 | TAGs (mmol/L) | −0.11 (0.000) | 17 | TAGs | −0.05 (NS) | 28 | TAGs (mmol/L) | −0.05 (NS) | 27 |
| SBP | −0.26 (0.012) | 14 | SBP (mm Hg) | −3.10 (0.033) | 11 | SBP | −0.25 (0.000) | 43 | SBP (mm Hg) | −1.54 (0.000) | 38 |
| DBP | −0.08 (NS) | 15 | DBP (mm Hg) | −0.55 (NS) | 14 | DBP | −0.22 (0.002) | 30 | DBP (mm Hg) | −1.62 (0.000) | 29 |
| FMD | +0.62 (0.014) | 3 | FMD (%) | +0.39 (NS) | 3 | FMD | −0.20 (NS) | 6 | FMD (%) | −0.65 (NS) | 6 |
| Glucose | −0.19 (0.058) | 14 | Glucose (mmol/L) | −0.18 (0.017) | 13 | Glucose | −0.13 (NS) | 22 | Glucose (mmol/L) | −0.02 (NS) | 20 |
| (a) | ET-Containing Products | ||||||||
| Source | Pomegranate | Nuts | |||||||
| SDM (p-Value) | n | DM (p-Value) | n | SDM (p-Value) | n | DM (p-Value) | n | Comparison between Subgroups (Q Statistic, p-Value) | |
| WC (cm) | −0.20 (NS) | 1 | −3.90 (NS) | 1 | −0.78 (0.027) | 6 | −1.51 (0.038) | 5 | SDM: 0.40, NS DM: 0.08, NS |
| T-C (mmol/L) | −0.04 (NS) | 11 | −0.02 (NS) | 11 | −0.32 (0.000) | 17 | −0.098 (0.000) | 15 | SDM: 10.83, 0.001 DM: 3.31, 0.069 |
| LDL-C (mmol/L) | −0.07 (NS) | 10 | −0.05 (NS) | 10 | −0.26 (0.047) | 16 | −0.11 (0.000) | 14 | SDM: 1.31, NS DM: 0.66, NS |
| HDL-C (mmol/L) | +0.11 (NS) | 10 | +0.01 (NS) | 10 | +0.14 (NS) | 13 | +0.03 (0.029) | 11 | SDM: 0.09, NS DM: 0.30, NS |
| TAGs (mmol/L) | −0.05 (NS) | 10 | −0.01 (NS) | 10 | −0.33 (0.031) | 16 | −0.11 (0.000) | 14 | SDM: 1.44, NS DM: 1.10, NS |
| SBP (mm Hg) | −0.09 (NS) | 8 | −0.26 (NS) | 6 | −0.13 (NS) | 13 | −1.63 (NS) | 9 | SDM: 0.03, NS DM: 0.03, NS |
| DBP (mm Hg) | −0.46 (0.000) | 8 | −4.31 (0.000) | 8 | +0.06 (NS) | 12 | +0.58 (0.004) | 10 | SDM: 12.95, 0.000 DM: 17.32, 0.000 |
| FMD (%) | +0.71 (NS) | 1 | +0.05 (NS) | 1 | +0.58 (0.058) | 2 | +1.04 (0.053) | 2 | SDM: 0.07, NS DM: 3.37, NS |
| Glucose (mmol/L) | −0.10 (NS) | 7 | −0.09 (NS) | 7 | −0.36 (0.079) | 8 | −0.14 (0.061) | 8 | SDM: 1.17, NS DM: 0.10, NS |
| (b) | ANC-Containing Products | ||||||||
| Source | Berries | Red Wine/Red Grapes | |||||||
| SDM (p-Value) | n | DM (p-Value) | n | SDM (p-Value) | n | DM (p-Value) | n | Comparison between Subgroups (Q Statistic, p-Value) | |
| T-C (mmol/L) | −0.21 (0.021) | 38 | −0.16 (0.093) | 35 | −0.14 (NS) | 44 | −0.06 (NS) | 43 | SDM: 0.30, NS DM: 0.70, NS |
| SBP (mm Hg) | −0.25 (0.000) | 38 | −2.41 (0.000) | 34 | −0.21 (0.000) | 36 | −3.31 (0.014) | 34 | SDM: 0.11, NS DM: 0.35, NS |
| DBP (mm Hg) | −0.25 (0.001) | 42 | −1.57 (0.002) | 37 | −0.16 (0.000) | 39 | −1.50 (0.002) | 35 | SDM: 0.80, NS DM: 0.06, NS |
| FMD (%) | +0.46 (NS) | 9 | +1.39 (0.011) | 8 | −0.19 (NS) | 10 | −0.73 (NS) | 10 | SDM: 2.89, NS DM: 5.68, NS |
| Hb1Ac | −0.63 (0.044) | 7 | −0.20 (0.040) | 6 | +0.97 (0.038) | 7 | +0.26 (0.026) | 7 | SDM: 8.59, 0.003 DM: 9.41, 0.002 |
| Factors | |||||
|---|---|---|---|---|---|
| Baseline BMI | 25.0 a (normal and (or) underweight) | ≥25.0 (overweight and (or) obese) | |||
| Sex | Women | Men | |||
| Smoking | Non-smokers | Smokers | |||
| Country where the study was conducted | East Asian countries (Japan, Korea, China) | All-other- countries-but-not-East Asian | North America (USA, Canada) | European countries | |
| Non-Mediterranean countries (Denmark, Norway, Finland, The Netherlands, Germany, Poland, UK, Scotland, France, Czech Republic) | Mediterranean countries (Italy, Spain, Greece) | ||||
| Medication | Yes | No | |||
| Health status | Healthy individuals b | Individuals ‘at a risk‘ of disease c | Individuals with a reported disease d | ||
| Main source of compounds | Ellagitannins | Anthocyannins | |||
| Pomegranate | Nuts | Berries | Red wine and red grapes | ||
| Diet during intervention | Controlled diet (specifically indicated to have restriction for the consumption of polyphenols or plant foods) | Usual diet (no changes in the usual diet of the participants or NR) | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Conesa, M.-T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andrés-Lacueva, C.; De Pascual-Teresa, S.; Mena, P.; Konic Ristic, A.; Hollands, W.J.; et al. Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses. Int. J. Mol. Sci. 2018, 19, 694. https://doi.org/10.3390/ijms19030694
García-Conesa M-T, Chambers K, Combet E, Pinto P, Garcia-Aloy M, Andrés-Lacueva C, De Pascual-Teresa S, Mena P, Konic Ristic A, Hollands WJ, et al. Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses. International Journal of Molecular Sciences. 2018; 19(3):694. https://doi.org/10.3390/ijms19030694
Chicago/Turabian StyleGarcía-Conesa, María-Teresa, Karen Chambers, Emilie Combet, Paula Pinto, Mar Garcia-Aloy, Cristina Andrés-Lacueva, Sonia De Pascual-Teresa, Pedro Mena, Aleksandra Konic Ristic, Wendy J. Hollands, and et al. 2018. "Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses" International Journal of Molecular Sciences 19, no. 3: 694. https://doi.org/10.3390/ijms19030694
APA StyleGarcía-Conesa, M. -T., Chambers, K., Combet, E., Pinto, P., Garcia-Aloy, M., Andrés-Lacueva, C., De Pascual-Teresa, S., Mena, P., Konic Ristic, A., Hollands, W. J., Kroon, P. A., Rodríguez-Mateos, A., Istas, G., Kontogiorgis, C. A., Rai, D. K., Gibney, E. R., Morand, C., Espín, J. C., & González-Sarrías, A. (2018). Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses. International Journal of Molecular Sciences, 19(3), 694. https://doi.org/10.3390/ijms19030694