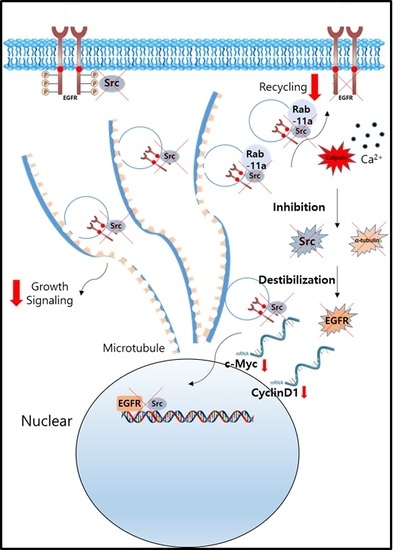
Antitumor Effect of Calcium-Mediated Destabilization of Epithelial Growth Factor Receptor on Non-Small Cell Lung Carcinoma
Abstract
:1. Introduction
2. Results
2.1. Src Is Destabilized in Non-Small Cell Lung Carcinoma (NSCLC) Cells after Lactate Calcium Salt (LCS) Treatment in a Time-Dependent Manner
2.2. LCS Treatment Destabilizes α-Tubulin in NSCLC Cells in a Time-Dependent Manner
2.3. EGFR Is Destabilized in NSCLC Cells Following LCS-Mediated Src and α-Tubulin Degradation
2.4. Destabilization of Src and EGFR by LCS Is Mediated by Calcium-Dependent Calpain Activation
2.5. Destabilization of α-Tubulin by LCS Is Mediated by Calcium-Dependent Calpain Activation
2.6. Nuclear Signaling in NSCLC Is Reduced Following Calcium-Mediated Destabilization of EGFR and Src
2.7. LCS Treatment Reduces Proliferation of NSCLC Cells
2.8. The In Vivo Antitumor Effect of LCS on NSCLC Is Induced by EGFR and Src Destabilization
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. Reagents
4.3. Protein Extraction
4.4. Western Blot Analysis
4.5. Immunocytochemistry
4.6. Colony Formation Assay
4.7. Animals
4.8. Xenograft Animal Model
4.9. Immunohistochemistry
4.10. Quantitative Reverse Transcription-Polymerase Chain Reaction
4.11. Statistical Analysis
Author Contributions
Conflicts of Interest
References
- Jung, K.W.; Won, Y.J.; Oh, C.M.; Kong, H.J.; Lee, D.H.; Lee, K.H. Prediction of Cancer Incidence and Mortality in Korea, 2017. Cancer Res. Treat. 2017, 49, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Fraguas, S.; Barberan, S.; Cebria, F. EGFR signaling regulates cell proliferation, differentiation and morphogenesis during planarian regeneration and homeostasis. Dev. Boil. 2011, 354, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, M.R.; Falcao, A.S.; Fuzii, H.T.; da Silva Kataoka, M.S.; Ribeiro, A.L.; Boccardo, E.; de Siqueira, A.S.; Jaeger, R.G.; de Jesus Viana Pinheiro, J.; de Melo Alves Junior, S. EGFR signaling downstream of EGF regulates migration, invasion, and MMP secretion of immortalized cells derived from human ameloblastoma. Tumour Boil. 2014, 35, 11107–11120. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Taylor, P.; Peterman, S.M.; Prakash, A.; Moran, M.F. Epidermal growth factor receptor phosphorylation sites Ser991 and Tyr998 are implicated in the regulation of receptor endocytosis and phosphorylations at Ser1039 and Thr1041. Mol. Cell. Proteom. 2009, 8, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, A.; von Zastrow, M. Endocytosis and signalling: Intertwining molecular networks. Nat. Rev. Mol. Cell Boil. 2009, 10, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, A.; Goh, L.K. Endocytosis and intracellular trafficking of ErbBs. Exp. Cell Res. 2009, 315, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pawson, T. Biochemistry of the Src protein-tyrosine kinase: Regulation by SH2 and SH3 domains. Recent Prog. Horm. Res. 1994, 49, 149–160. [Google Scholar] [PubMed]
- Chung, B.M.; Tom, E.; Zutshi, N.; Bielecki, T.A.; Band, V.; Band, H. Nexus of signaling and endocytosis in oncogenesis driven by non-small cell lung cancer-associated epidermal growth factor receptor mutants. World J. Clin. Oncol. 2014, 5, 806–823. [Google Scholar] [CrossRef] [PubMed]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar] [PubMed]
- Bao, J.; Gur, G.; Yarden, Y. Src promotes destruction of c-Cbl: Implications for oncogenic synergy between Src and growth factor receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 2438–2443. [Google Scholar] [CrossRef] [PubMed]
- Gunning, P.W.; Ghoshdastider, U.; Whitaker, S.; Popp, D.; Robinson, R.C. The evolution of compositionally and functionally distinct actin filaments. J. Cell Sci. 2015, 128, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sooman, L.; Lennartsson, J.; Bergstrom, S.; Bergqvist, M.; Gullbo, J.; Ekman, S. Microtubule inhibition causes epidermal growth factor receptor inactivation in oesophageal cancer cells. Int. J. Oncol. 2013, 42, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs that target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.S.; Jo, Y.K.; Sim, J.J.; Ji, E.; Jeong, K.Y.; Kim, H.M. Lactate calcium salt affects the viability of colorectal cancer cells via betaine homeostasis. Life Sci. 2016, 147, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Sundaramoorthy, P.; Sim, J.J.; Jeong, K.Y.; Kim, H.M. Synergistically Anti-metastatic Effect of 5-Flourouracil on Colorectal Cancer Cells via Calcium-mediated Focal Adhesion Kinase Proteolysis. Anticancer Res. 2017, 37, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.Y.; Park, M.; Kim, I.U.; Sim, J.J.; Kim, H.M. Enhancing 5-Fluorouracil Efficacy in a Primary Colorectal Cancer by Long-lasting Calcium Supplementation. Anticancer Res. 2017, 37, 2959–2964. [Google Scholar] [PubMed]
- Tomas, A.; Futter, C.E.; Eden, E.R. EGF receptor trafficking: Consequences for signaling and cancer. Trends Cell Boil. 2014, 24, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Brand, T.M.; Iida, M.; Li, C.; Wheeler, D.L. The nuclear epidermal growth factor receptor signaling network and its role in cancer. Discov. Med. 2011, 12, 419–432. [Google Scholar] [PubMed]
- Ioannou, M.S.; McPherson, P.S. Rab-mediated membrane trafficking and the control of epithelial cell polarity. J. Cell Boil. 2016, 213, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Chua, B.T.; Guo, K.; Li, P. Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J. Boil. Chem. 2000, 275, 5131–5135. [Google Scholar] [CrossRef]
- Lwin, Z.; Riess, J.W.; Gandara, D. The continuing role of chemotherapy for advanced non-small cell lung cancer in the targeted therapy era. J. Thorac. Dis. 2013, 5 (Suppl. S5), S556–S564. [Google Scholar] [PubMed]
- Reinecke, J.; Caplan, S. Endocytosis and the Src family of non-receptor tyrosine kinases. Biomol. Concepts 2014, 5, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar]
- Sato, K. Cellular functions regulated by phosphorylation of EGFR on Tyr845. Int. J. Mol. Sci. 2013, 14, 10761–10790. [Google Scholar] [CrossRef] [PubMed]
- Granger, E.; McNee, G.; Allan, V.; Woodman, P. The role of the cytoskeleton and molecular motors in endosomal dynamics. Semin. Cell Dev. Biol. 2014, 31, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Deribe, Y.L.; Wild, P.; Chandrashaker, A.; Curak, J.; Schmidt, M.H.; Kalaidzidis, Y.; Milutinovic, N.; Kratchmarova, I.; Buerkle, L.; Fetchko, M.J.; et al. Regulation of epidermal growth factor receptor trafficking by lysine deacetylase HDAC6. Sci. Signal. 2009, 2, ra84. [Google Scholar] [PubMed]
- Mathiasen, I.S.; Sergeev, I.N.; Bastholm, L.; Elling, F.; Norman, A.W.; Jaattela, M. Calcium and calpain as key mediators of apoptosis-like death induced by vitamin D compounds in breast cancer cells. J. Boil. Chem. 2002, 277, 30738–30745. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Orrenius, S.; Zhivotovsky, B. Serine proteases and calpains fulfill important supporting roles in the apoptotic tragedy of the cellular opera. Cell Death Differ. 2005, 12, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Jagoe, W.N.; Lindsay, A.J.; Read, R.J.; McCoy, A.J.; McCaffrey, M.W.; Khan, A.R. Crystal structure of rab11 in complex with rab11 family interacting protein 2. Structure 2006, 14, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Marie, N.; Lindsay, A.J.; McCaffrey, M.W. Rab coupling protein is selectively degraded by calpain in a Ca2+-dependent manner. Biochem. J. 2005, 389 Pt 1, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Baldin, V.; Lukas, J.; Marcote, M.J.; Pagano, M.; Draetta, G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993, 7, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and cancer metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef] [PubMed]
- McKeown, M.R.; Bradner, J.E. Therapeutic strategies to inhibit MYC. Cold Spring Harb. Perspect. Med. 2014, 4, a014266. [Google Scholar] [CrossRef] [PubMed]
- Gautschi, O.; Ratschiller, D.; Gugger, M.; Betticher, D.C.; Heighway, J. Cyclin D1 in non-small cell lung cancer: A key driver of malignant transformation. Lung Cancer 2007, 55, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.A.; Hughes, B.G. Targeted therapy for non-small cell lung cancer: Current standards and the promise of the future. Transl. Lung Cancer Res. 2015, 4, 36–54. [Google Scholar] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih image to imagej: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]









| Gene | Direction | Primer Sequence |
|---|---|---|
| Actin | Sense | GGTTCACTTTTT-CAAGCAGTAGG |
| Anti-sense | GTGGTAATCCACTTTCATCCATT | |
| c-Myc | Sense | AAC-TGGAACGGTGAAGGT |
| Anti-sense | CCTGTAACAACGCATCTCAT | |
| Cyclin D1 | Sense | ACA-TCTTCCAGGAGTACCC |
| Anti-sense | CTTGGTGAGGTTTGATCCG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.U.; Sung, I.S.; Sim, J.J.; Park, M.; Jeong, K.-Y.; Kim, H.M. Antitumor Effect of Calcium-Mediated Destabilization of Epithelial Growth Factor Receptor on Non-Small Cell Lung Carcinoma. Int. J. Mol. Sci. 2018, 19, 1158. https://doi.org/10.3390/ijms19041158
Kim IU, Sung IS, Sim JJ, Park M, Jeong K-Y, Kim HM. Antitumor Effect of Calcium-Mediated Destabilization of Epithelial Growth Factor Receptor on Non-Small Cell Lung Carcinoma. International Journal of Molecular Sciences. 2018; 19(4):1158. https://doi.org/10.3390/ijms19041158
Chicago/Turabian StyleKim, In Un, In Sung Sung, Jae Jun Sim, Minhee Park, Keun-Yeong Jeong, and Hwan Mook Kim. 2018. "Antitumor Effect of Calcium-Mediated Destabilization of Epithelial Growth Factor Receptor on Non-Small Cell Lung Carcinoma" International Journal of Molecular Sciences 19, no. 4: 1158. https://doi.org/10.3390/ijms19041158
APA StyleKim, I. U., Sung, I. S., Sim, J. J., Park, M., Jeong, K.-Y., & Kim, H. M. (2018). Antitumor Effect of Calcium-Mediated Destabilization of Epithelial Growth Factor Receptor on Non-Small Cell Lung Carcinoma. International Journal of Molecular Sciences, 19(4), 1158. https://doi.org/10.3390/ijms19041158