Detrimental Effects of Helium Ion Irradiation on Cognitive Performance and Cortical Levels of MAP-2 in B6D2F1 Mice
Abstract
:1. Introduction
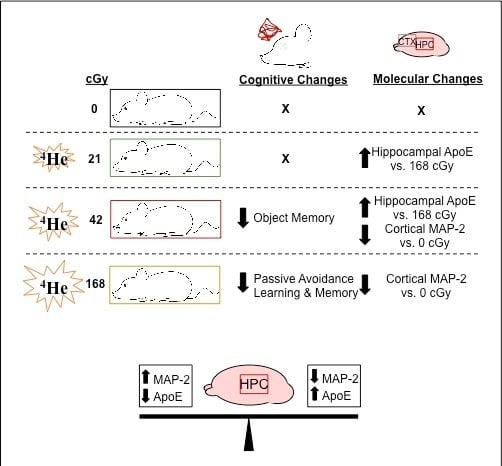
2. Results
2.1. Behavioral Performance in the Open Field and Object Recognition
2.2. Depressive-Like Behavior in the Forced Swim Test and Contextual and Cued Fear Memory
2.3. Passive Avoidance Learning and Memory
2.4. MAP-2, CD68, BDNF and ApoE Levels in the Cortex and Hippocampus
3. Discussion
4. Materials and Methods
4.1. Animals and Study Design
4.2. Behavioral and Cognitive Testing
4.3. Exploratory Behavior in the Open Field and Object Recognition (Week 1)
4.4. Depression-Like Behavior and Contextual and Cued Fear Learning and Memory (Week 2)
4.5. Passive Avoidance Learning and Memory (Week 3)
4.6. MAP-2, CD68, BDNF and ApoE ELISAs
4.7. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cucinotta, F.A.; Kim, M.; Willigham, V.; George, K.A. Physical and biological organ dosimetry analysis for International Space Station Astronauts. Radiat. Res. 2008, 170, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, C.; Hassler, D.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.; Brinza, D.; Kang, S.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Measurements of energetic particle radiation in transit to mars on the mars science laboratoryy. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Hassler, D.M.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Ehresmann, B.; Rafkin, S.; Eigenbrode, J.L.; Brinza, D.E.; Weigle, G.; Böttcher, S.; Böhm, E.; et al. Mars’ surface radiation environment measured with the Mars Science Laboratory’s Curiosity rover. Science 2014, 343, 1244797. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Raber, J.; Chakraborti, A.; Sharma, S.; Fike, J.R. 56Fe irradiation alters spine density and dendritic complexity in the mouse hippocampus. Radiat. Res. 2015, 184, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Haley, G.E.; Villasana, L.; Dayger, C.; Davis, M.J.; Raber, J. ApoE Genotype-Dependent Paradoxical Short-Term Effects of 56Fe Irradiation on the Brain. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Allen, A.; Rosi, S.; Sharma, S.; Dayger, C.; Davis, M.; Fike, J.R. Effects of 56Fe radiation on hippocampal function in mice deficient in chemokine receptor 2 (CCR2). Behav. Brain Res. 2013, 246, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Allen, A.; Rosi, S.; Sharma, S.; Dayger, C.; Davis, M.; Fike, J.R. Effects of whole body 56Fe radiation on contextual freezing and Arc-positive cells in the dentate gyrus. Behav. Brain Res. 2012, 246, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Rabin, B.; Buhler, L.; Joseph, J.; Shukitt-Hale, B.; Jenkins, D. Effects of exposure to 56Fe particles or protons on fixed ratio operant responding in rats. J. Radiat. Res. 2002, 4, S225–S228. [Google Scholar] [CrossRef]
- Rabin, B.; Carrhill-Knoll, K.; Carey, A.; Shukitt-Hale, B.; Joseph, J.; Foster, B. Elevated plus-maze performance of Fischer-244 rats as a function of age and exposure to 56Fe particles. Adv. Space Res. 2007, 39, 981–986. [Google Scholar] [CrossRef]
- Britten, R.; Davis, L.; Johnson, A.; Keeney, S.; Siegel, A.; Sanford, L.; Singletary, S.; Lonart, G. Low (20 cGy) doses of 1 GeV/u 56Fe-particle radiation lead to a persistent reduction in the spatial learning ability of rats. Radiat. Res. 2012, 177, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Denisova, N.A.; Shukitt-Hale, B.; Rabin, B.M.; Joseph, J.A. Brain signaling and behavioral responses induced by exposure to (56)Fe-particle radiation. Radiat. Res. 2002, 158, 725–734. [Google Scholar] [CrossRef]
- Raber, J.; Marzulla, T.; Stewart, B.; Kronenberg, A.; Turker, M.S. (28)Silicon Irradiation Impairs Contextual Fear Memory in B6D2F1 Mice. Radiat. Res. 2015, 183, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Rudobeck, E.; Allen, A.; Allen, B.; Rosi, S.; Nelson, G.; Ramachandran, S.; Turner, J.; Fike, J.; Vlkolinsky, R. 28Silicon radiation-induced enhancement of synaptic plasticity in the hippocampus of naive and cognitively tested mice. Radiat. Res. 2014, 181, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Vlkolinsky, R.; Campbell-Beachler, M.; Sokolova, I.; Obenaus, A.; Nelson, G. Functional effects of proton, silicon, and iron radiation on synaptic excitability in the mouse hippocampus. In Proceedings of the 23rd Annual Space Radiation Investgators’ Workshop, Durham, NC, USA, 8–12 July 2012. [Google Scholar]
- Bellone, J.; Hartman, R.; Vlkolinsky, R. The effects of low doses of proton, iron, or silicon radiation on spatial learning in a mouse model of Alzheimer’s disease. J. Radiat. Res. 2014, 55 (Suppl. S1), i95–i96. [Google Scholar] [CrossRef]
- Raber, J.; Weber, S.J.; Kronenberg, A.; Turker, M.S. Sex- and dose-dependent effects of calcium ion irradiation on behavioral performance of B6D2F1 mice during contextual fear conditioning training. Life Sci. Space Res. 2016, 9, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Marzulla, T.; Kronenberg, A.; Turker, M.S. 16Oxygen irradiation enhances cued fear memory in B6D2F1 mice. Life Sci. Space Res. 2015, 7, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.H.; DeLaPaz, R.; Frankel, K.A.; Poljak, A.; Phillips, M.H.; Brennan, K.M.; Woodruff, K.H.; Valk, P.E.; Steinberg, G.K.; Fabrikant, J.I. MRI and PET of delayed heavy-ion radiation injury in the rabbit brain. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 689–696. [Google Scholar] [CrossRef]
- Linstadt, D.; Castro, J.; Char, D.; Decker, M.; Ahn, D.R.; Petti, P.; Nowakowski, V.; Quivey, J.; Phillips, T.J. Long-term results of helium ion irradiation of uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 613–618. [Google Scholar] [CrossRef]
- Philliips, T.; Fu, K.F.; Curtis, C.B. Tumor biology of helium and heavy ions. Int. J. Radiat. Oncol. Biol. Phys. 1977, 3, 109–113. [Google Scholar] [CrossRef]
- Rabin, B.; Joseph, J.; Hunt, W.; Dalton, T.; Kandasamy, S.; Harris, A.; Ludewight, B. Behavioral endpoints for radiation injury. Adv. Space Res. 1994, 14, 457–466. [Google Scholar] [CrossRef]
- Kandasamy, S.; Rabin, B.; Hunt, W.; Dalton, T.; Joseph, J.; Harris, A. Exposure to heavy charged particles affects thermoregulation in rats. Radiat. Res. 1994, 139, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Rabin, B.; Hunt, W.; Joseph, J.; Dalton, T.; Kandasamy, S. Relationship between linear energy transfer and behavioral toxicity in rats following exposure to protons and heavy particles. Radiat. Res. 1991, 128, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Rabin, B.; Carrihill-Knoll, K.; Shukitt-Hale, B. Comparison of the effectiveness of exposure to low-LET helium particles (4He) and gamma rays (137Cs) on the disruption of cognitive performance. Radiat. Res. 2015, 184, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Stuster, J. Behavioral Issues Associated with Long Duration Space Expeditions: Review and Analysis of Astronaut Journals Experiment 01-E104 (Journals) Phase 2 Final Report; NASA Technical Memorandum; NASA: Houston, TX, USA, 2016.
- Raber, J.; Allen, A.R.; Sharma, S.; Allen, B.; Rosi, S.; Olsen, R.H.J.; Davis, M.J.; Eiwaz, M.; Fike, J.R.; Nelson, G.A. Effects of proton and combined proton and 56Fe irradiation on the hippocampus. Radiat. Res. 2015, 184, 586–594. [Google Scholar]
- Impey, S.; Jopson, T.; Pelz, C.; Tafessu, A.; Fareh, F.; Zuloaga, D.; Marzulla, T.; Riparip, L.-L.; Stewart, B.; Rosi, S.; et al. Short- and long-term effects of 56Fe irradiation on cognition and hippocampal DNA methylation and gene expression. BMC Genom. 2016, 17, 825. [Google Scholar] [CrossRef] [PubMed]
- Villasana, L.; Rosenberg, J.; Raber, J. Sex-dependent effects of 56Fe Irradiation on contextual fear conditioning in C56BL/6J mice. Hippocampus 2010, 20, 19–23. [Google Scholar] [PubMed]
- Rabin, B.; Shukitt-Hale, B.; Carrihill-Knoll, K. Effects of age on disruption of cognitive performance by exposure to space radiation. J. Behav. Brain Sci. 2014, 4, 297–307. [Google Scholar] [CrossRef]
- Harada, A.; Teng, J.; Takei, Y.; Oguchi, K.; Hirokawa, N. MAP-2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J. Cell Biol. 2002, 158, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benice, T.; Rizk, A.; Pfankuch, T.; Kohama, S.; Raber, J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience 2006, 137, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Peister, A.; Zeitouni, S.; Pfankuch, T.; Prockop, D.; Raber, J. Novel object recognition in Apoe−/− mice improved by neonatal implantation of wildtype multipotent stromal cells. Exp. Neurol. 2006, 201, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Haley, G.; Kohama, S.; Urbanski, H.; Raber, J. Age-related decreases in SYN levels associated with increases in MAP-2, apoE, and GFAP levels in the rhesus nacaque prefrontal cortex and hippocampus. AGE 2010, 32, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.; Marzulla, T.; Raber, J. Impairment in extinction of contextual and cued fear following post-training whole body irradiation. Frontiers 2014, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Villasana, L.; Pfankuch, T.; Raber, J. Isoform-Dependent Effects of apoE on Doublecortin-Positive Cells and Microtubule-Associated Protein 2 Immunoreactivity following 137Cs Irradiation. Radiat. Environ. Biophys. 2010, 49, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Lu, Y.; Sun, R.; Ji, J.; Zhang, L.; Duan, S. Irradiation-induced hippocampal neurogenesis impairment is associated with epigenetic regulation of bdnf gene transcription. Brain Res. 2014, 1577, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Yang, M.; Kang, S.; Lee, S.; Kim, J.-S.; Park, S.; Kim, J.; Jo, S.; Jung, U.; Shin, T.; et al. Cranial irradiation regulates CREB-BDNF signaling and variant BDNF transcript levels in the mouse hippocampus. Neurobiol. Learn. Mem. 2015, 121, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, C.; Yang, G.; Ninan, I.; Savas, J.; Yates, J.; Lafaille, J.; Hempstead, B.; Littman, D.R.; Gan, W.B. Microglia promote learning-dependent synapse formation through BDNF. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Ferreira, R.; George, J.; Sanches, R.; Rodrigues, D.; Goncalves, N.; Cunha, R. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J. Neuroinflammation 2013, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, L.; Yu, T.; Xu, Y.; Pu, S.J.; Du, D.; Jiang, W.W. Positive feedback loop of autocrine BDNF from microglia causes prolonged microglia activation. Cell. Physiol. Biochem. 2014, 34, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, Y.; Kato, T.; Seki, Y.; Ohgidani, M.; Sagata, N.; Horikawa, H.; Yamauchi, Y.; Sato-Kasai, M.; Hayakawa, K.; Inoue, R.; et al. Brain-derived Neurotrophic Factor (BDNF) Induces Sustained Intracellular Ca2+ Elevation through the Up-regulation of Surface Transient Receptor Potential 3 (TRPC3) Channels in Rodent Microglia. J. Biol. Chem. 2014, 289, 18549–18555. [Google Scholar] [CrossRef] [PubMed]
- Neuen-Jacob, E.; Arendt, G.; Wendtland, B.; Jacob, B.; Schneeweis, M.; Wechsler, W. Frequency and topographical distribution of CD68-positive macrophages and HIV-1 core proteins in HIV-associated brain lesions. Clin. Neuropathol. 1993, 12, 315–324. [Google Scholar] [PubMed]
- Tanaka, Y.; Matsuwaki, T.; Yamaouchi, K.; Nishihara, M. Exacerbated inflammatory responses related to activated microglia after traumatic brain injury in progranulin-deficient mice. Neuroscience 2013, 231, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Chakraborti, A.; Eilertson, K.; Sharma, S.; Baure, J.; Habdank-Kolaczkowski, J.; Allen, B.; Rosi, S.; Raber, J.; Fike, J.R. Radiation exposure to juvenile mice induces a heightened sensitivity to traumatic brain injury in adulthood. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.; Patel, N.; Craver, B.; Tran, K.; Giedzinski, E.; Tseng, B.; Parihar, V.; Limoli, C.L. Consequences of Low Dose Ionizing Radiation Exposure on the Hippocampal Microenvironment. PLoS. ONE 2015, 10, e0128316. [Google Scholar] [CrossRef] [PubMed]
- Parihar, V.; Allen, B.; Caressi, C.; Kwok, S.; Chu, E.; Tran, K.; Chmielewski, N.; Giedzinski, E.; Acharya, M.; Britten, R.; et al. Cosmic radiation exposure and persistent cognitive dysfunction. Sci. Rep. 2016, 6, 34774. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y.; Nelson, G.A.; Vazquez, M.; Laskowitz, D.T.; Slater, J.M.; Pearlstein, R.D. Apolipoprotein E expression and behavioral toxicity of high charge, high energy (HZE) particle radiation. J. Radiat. Res. 2002, 43, S219–S224. [Google Scholar] [CrossRef] [PubMed]
- Villasana, L.; Benice, T.; Raber, J. Long-term effects of 56Fe irradiation on spatial memory of mice: Role of sex and apolipoprotein E isoform. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Slaba, T.; Blattnig, S.; Norbury, J.; Rusek, A.; La Tessa, C. Reference field specification and preliminary beam selection strategy for accelerator-based GCR stimulation. Life Sci. Space Res. 2016, 8, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Raber, J. Novel images and novel locations of familiar images as sensitive translational cognitive tests in humans. Behav. Brain Res. 2015, 285, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Mineka, S. The role of fear in theories of avoidance learning, flooding, and extinction. Psychol. Bull. 1979, 86, 985–1010. [Google Scholar] [CrossRef]
- Cornwell, B.R.; Overstree, C.; Krimsky, M.; Grillon, C. Passive avoidance is linked to impaired fear extinction in humans. Learn. Mem. 2013, 20, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Stackman, R. Assessing rodent hippocampal involvement in the novel object recognition task: A review. Behav. Brain Res. 2014, 285, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, S. Retrograde and antergrade object recognition in rats with hippocampal lesions. Hippocampus 2003, 13, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Winters, B.D.; Forwood, S.E.; Cowell, R.A.; Saksida, L.M.; Bussey, T.J. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. J. Neurosci. 2004, 24, 5901–5908. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, C.; Baldi, E.; Bucherelli, C.; Saccheti, B.; Tassoni, G. Role of ventral hippocampus in acquisition, consolidation and retrieval of rat’s passive avoidance response memory trace. Brain Res. 1997, 768, 242–248. [Google Scholar] [CrossRef]
- Baarendse, P.J.; van Grootheest, G.; Jansen, R.F.; Pieneman, A.W.; Ogren, S.O.; Verhage, M.; Stiedl, O. Differential involvement of the dorsal hippocampus in passive avoidance in C57bl/6J and DBA/2J mice. Hippocampus 2008, 18, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Dunn, T.J.; Lindahl, R.; Pitot, H.C. Differential gene expression in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). J. Biol. Chem. 1988, 263, 10878–10886. [Google Scholar] [PubMed]
- Cucinotta, F.A. Review of NASA approach to space radiation risk assessments for Mars exploration. Health Phys. 2015, 108, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Engle, S.J.; Womer, D.E.; Davies, P.M.; Boivin, G.; Sahota, A.; Simmonds, H.A.; Stambrook, P.J.; Tischfield, J.A. HPRT-APRT-deficient mice are not a model for lesch-nyhan syndrome. Hum. Mol. Genet. 1996, 5, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, A.; Gauny, S.; Kwoh, E.; Connolly, L.; Dan, C.; Lasarev, M.; Turke, R.M. Comparative analysis of cell killing and autosomal mutation in mouse kidney epithelium exposed to 1 GeV/nucleon iron ions in vitro or in situ. Radiat. Res. 2009, 172, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A. One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav. Brain Res. 2010, 215, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, R.; Shen, J.; Su, H.; Xing, D.; Du, L. A mouse model of depression induced by repeated corticosterone injections. Eur. J. Pharmacol. 2008, 581, 113–129. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, G.J.; Friedman, D.; Young, K.H.; Torres, E.R.; Thomas, C.R., Jr.; Gough, M.J.; Raber, J. Neuroinflammatory and cognitive consequences of combined radiation and immunotherapy in a novel preclinical model. Oncotarget 2017, 8, 9155–9173. [Google Scholar] [CrossRef] [PubMed]
- Maren, S. Neurobiology of Pavlovian fear conditioning. Ann. Rev. Neurosci. 2001, 24, 897–931. [Google Scholar] [CrossRef] [PubMed]
- Anagnostaras, S.; Gale, G.; Franselow, M. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus 2001, 11, 8–17. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raber, J.; Torres, E.R.S.; Akinyeke, T.; Lee, J.; Weber Boutros, S.J.; Turker, M.S.; Kronenberg, A. Detrimental Effects of Helium Ion Irradiation on Cognitive Performance and Cortical Levels of MAP-2 in B6D2F1 Mice. Int. J. Mol. Sci. 2018, 19, 1247. https://doi.org/10.3390/ijms19041247
Raber J, Torres ERS, Akinyeke T, Lee J, Weber Boutros SJ, Turker MS, Kronenberg A. Detrimental Effects of Helium Ion Irradiation on Cognitive Performance and Cortical Levels of MAP-2 in B6D2F1 Mice. International Journal of Molecular Sciences. 2018; 19(4):1247. https://doi.org/10.3390/ijms19041247
Chicago/Turabian StyleRaber, Jacob, Eileen Ruth S. Torres, Tunde Akinyeke, Joanne Lee, Sydney J. Weber Boutros, Mitchell S. Turker, and Amy Kronenberg. 2018. "Detrimental Effects of Helium Ion Irradiation on Cognitive Performance and Cortical Levels of MAP-2 in B6D2F1 Mice" International Journal of Molecular Sciences 19, no. 4: 1247. https://doi.org/10.3390/ijms19041247
APA StyleRaber, J., Torres, E. R. S., Akinyeke, T., Lee, J., Weber Boutros, S. J., Turker, M. S., & Kronenberg, A. (2018). Detrimental Effects of Helium Ion Irradiation on Cognitive Performance and Cortical Levels of MAP-2 in B6D2F1 Mice. International Journal of Molecular Sciences, 19(4), 1247. https://doi.org/10.3390/ijms19041247