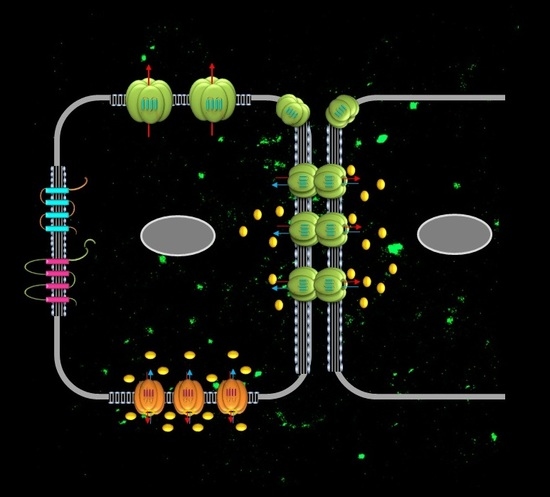
Connexin Communication Compartments and Wound Repair in Epithelial Tissue
Abstract
:1. Introduction
2. Connexins in Normal and Repairing Airway Epithelium
2.1. Connexins in the Airway Epithelium
2.2. Repair Research in the Adult Airway Epithelium
2.3. Connexins in Wound Repair of the Airway Epithelium
3. Connexins in the Epidermis
3.1. Connexins in Epidermal Wound Healing
3.2. Connexins and Inflammation in the Epidermis: Chronic Wounds and Psoriasis
4. Disruption of Cx43:Cx26 Balance in Epithelial Tissue: Connexins and the Environment
5. Connexins as Therapeutic Targets in Epithelial Tissues
6. Zebrafish Connexins in Wound Repair and Regeneration
6.1. Wound-Repair Research in Zebrafish
6.2. Zebrafish Connexins
6.3. Zebrafish Connexins in the Heart
6.4. Zebrafish Connexins in the Fin
7. Concluding Remarks
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| 15kPGE2 | 15-keto prostaglandin E2 |
| ATP | adenosine triphosphate |
| BC | basal cell |
| CC | ciliated cell |
| CF | cystic fibrosis |
| CK | cytokeratin |
| CRISPR-cas9 | clustered regularly interspaced short palindromic repeats-Cas9 |
| Cx | connexin |
| ECM | extracellular matrix |
| EP | early progenitor cell |
| GC | goblet /secretory cell |
| HPGD | hydroxyprostaglandin dehydrogenase |
| KO | knockout |
| LPS | lipopolysaccharide |
| Panx | pannexin |
| PGN | peptidoglycan |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| TGF-β | transforming growth factor β |
| Zf | zebrafish |
References
- Laird, D.W. Life cycle of connexins in health and disease. Biochem. J. 2006, 394, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.H.; Martin, P.E. Gap junctions: Structure and function (Review). Mol. Membr. Biol. 2002, 19, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Koval, M.; Isakson, B.E.; Gourdie, R.G. Connexins, Pannexins and innexins: Protein cousins with overlapping functions. FEBS Lett. 2014, 588, 1185. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Jin, X.; Medina, C.B.; Leonhardt, S.A.; Kiessling, V.; Bennett, B.C.; Shu, S.; Tamm, L.K.; Yeager, M.; Ravichandran, K.S.; et al. A quantized mechanism for activation of pannexin channels. Nat. Commun. 2017, 8, 14324. [Google Scholar] [CrossRef] [PubMed]
- Bhalla-Gehi, R.; Penuela, S.; Churko, J.M.; Shao, Q.; Laird, D.W. Pannexin1 and pannexin3 delivery, cell surface dynamics, and cytoskeletal interactions. J. Biol. Chem. 2010, 285, 9147–9160. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.J.; Weber, P.A.; Cao, F.; Chang, H.; Lampe, P.; Goldberg, G. The molecular basis of selective permeability of connexins is complex and includes both size and charge. Braz. J. Med. Biol. Res. 2000, 33, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Ek-Vitorin, J.F.; Burt, J.M. Structural basis for the selective permeability of channels made of communicating junction proteins. Biochim. Biophys. Acta 2013, 1828, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W.; Naus, C.C.; Lampe, P.D. SnapShot: Connexins and disease. Cell 2017, 170, 1260. [Google Scholar] [CrossRef] [PubMed]
- Crespo Yanguas, S.; Willebrords, J.; Maes, M.; da Silva, T.C.; Veloso Alves Pereira, I.; Cogliati, B.; Zaidan Dagli, M.L.; Vinken, M. Connexins and pannexins in liver damage. EXCLI J. 2016, 15, 177–186. [Google Scholar] [PubMed]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.N.; Koval, M. Cross-talk between pulmonary injury, oxidant stress, and gap junctional communication. Antioxid. Redox Signal. 2009, 11, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Losa, D.; Chanson, M. The lung communication network. Cell. Mol. Life Sci. 2015, 72, 2793–2808. [Google Scholar] [CrossRef] [PubMed]
- Scheckenbach, K.E.; Crespin, S.; Kwak, B.R.; Chanson, M. Connexin channel-dependent signaling pathways in inflammation. J. Vasc. Res. 2011, 48, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Boitano, S.; Dirksen, E.R.; Sanderson, M.J. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science 1992, 258, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Droguett, K.; Rios, M.; Carreno, D.V.; Navarrete, C.; Fuentes, C.; Villalon, M.; Barrera, N.P. An autocrine ATP release mechanism regulates basal ciliary activity in airway epithelium. J. Physiol. 2017, 595, 4755–4767. [Google Scholar] [CrossRef] [PubMed]
- Shishikura, Y.; Koarai, A.; Aizawa, H.; Yamaya, M.; Sugiura, H.; Watanabe, M.; Hashimoto, Y.; Numakura, T.; Makiguti, T.; Abe, K.; et al. Extracellular ATP is involved in dsRNA-induced MUC5AC production via P2Y2R in human airway epithelium. Respir. Res. 2016, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.F.; Zhang, L.; Kreda, S.M.; Abdullah, L.H.; Davis, C.W.; Pickles, R.J.; Lazarowski, E.R.; Boucher, R.C. Coupled nucleotide and mucin hypersecretion from goblet-cell metaplastic human airway epithelium. Am. J. Respir. Cell Mol. Biol. 2011, 45, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Losa, D.; Kohler, T.; Bellec, J.; Dudez, T.; Crespin, S.; Bacchetta, M.; Boulanger, P.; Hong, S.S.; Morel, S.; Nguyen, T.H.; et al. Pseudomonas aeruginosa-induced apoptosis in airway epithelial cells is mediated by gap junctional communication in a JNK-dependent manner. J. Immunol. 2014, 192, 4804–4812. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.J.; Prince, A.S. TLR2 regulates gap junction intercellular communication in airway cells. J. Immunol. 2008, 180, 4986–4993. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Chang, G.; Hu, R.; Phillips, A.; Douglas, R. Connexin gap junction channels and chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2016, 6, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.; Ringholz, F.; Buchanan, P.; McNally, P.; Urbach, V. Physiological impact of abnormal lipoxin A(4) production on cystic fibrosis airway epithelium and therapeutic potential. BioMed Res. Int. 2015, 2015, 781087. [Google Scholar] [CrossRef] [PubMed]
- Molina, S.A.; Stauffer, B.; Moriarty, H.K.; Kim, A.H.; McCarty, N.A.; Koval, M. Junctional abnormalities in human airway epithelial cells expressing F508del CFTR. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L475–L487. [Google Scholar] [CrossRef] [PubMed]
- Ashino, Y.; Ying, X.; Dobbs, L.G.; Bhattacharya, J. [Ca(2+)](i) oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L5–L13. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, J.; Nickles, H.T.; Ranke, H.; Tabuchi, A.; Hoffmann, J.; Tabeling, C.; Barbosa-Sicard, E.; Chanson, M.; Kwak, B.R.; et al. Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J. Clin. Investig. 2012, 122, 4218–4230. [Google Scholar] [CrossRef] [PubMed]
- Chadjichristos, C.E.; Scheckenbach, K.E.; van Veen, T.A.; Richani Sarieddine, M.Z.; de Wit, C.; Yang, Z.; Roth, I.; Bacchetta, M.; Viswambharan, H.; Foglia, B.; et al. Endothelial-specific deletion of connexin40 promotes atherosclerosis by increasing CD73-dependent leukocyte adhesion. Circulation 2010, 121, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Sarieddine, M.Z.; Scheckenbach, K.E.; Foglia, B.; Maass, K.; Garcia, I.; Kwak, B.R.; Chanson, M. Connexin43 modulates neutrophil recruitment to the lung. J. Cell. Mol. Med. 2009, 13, 4560–4570. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, K.; Gusarova, G.A.; Islam, M.N.; Subramanian, M.; Cohen, T.S.; Prince, A.S.; Bhattacharya, J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 2014, 506, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.L.; Barkauskas, C.E.; Chapman, H.A.; Epstein, J.A.; Jain, R.; Hsia, C.C.; Niklason, L.; Calle, E.; Le, A.; Randell, S.H.; et al. Repair and regeneration of the respiratory system: Complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 2014, 15, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.K.; Rulands, S.; Wilkinson, A.C.; Wuidart, A.; Ousset, M.; van Keymeulen, A.; Gottgens, B.; Blanpain, C.; Simons, B.D.; Rawlins, E.L. Clonal dynamics reveal two distinct populations of basal cells in slow-turnover airway epithelium. Cell Rep. 2015, 12, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Morrisey, E.E.; Cardoso, W.V.; Lane, R.H.; Rabinovitch, M.; Abman, S.H.; Ai, X.; Albertine, K.H.; Bland, R.D.; Chapman, H.A.; Checkley, W.; et al. Molecular determinants of lung development. Ann. Am. Thorac. Soc. 2013, 10, S12–S16. [Google Scholar] [CrossRef] [PubMed]
- Marcet, B.; Chevalier, B.; Coraux, C.; Kodjabachian, L.; Barbry, P. MicroRNA-based silencing of Delta/Notch signaling promotes multiple cilia formation. Cell Cycle 2011, 10, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Fallon, T.R.; Saladi, S.V.; Pardo-Saganta, A.; Villoria, J.; Mou, H.; Vinarsky, V.; Gonzalez-Celeiro, M.; Nunna, N.; Hariri, L.P.; et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev. Cell 2014, 30, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.E.; Mori, M.; Szymaniak, A.D.; Varelas, X.; Cardoso, W.V. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev. Cell 2014, 30, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Reed, W.; Moats-Staats, B.M.; Brighton, L.E.; Gambling, T.M.; Hu, S.C.; Collier, A.M. Connexin 26 expression in human and ferret airways and lung during development. Am. J. Respir. Cell Mol. Biol. 1998, 18, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Wiszniewski, L.; Sanz, J.; Scerri, I.; Gasparotto, E.; Dudez, T.; Lacroix, J.S.; Suter, S.; Gallati, S.; Chanson, M. Functional expression of connexin30 and connexin31 in the polarized human airway epithelium. Differentiation 2007, 75, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Crespin, S.; Bacchetta, M.; Bou Saab, J.; Tantilipikorn, P.; Bellec, J.; Dudez, T.; Nguyen, T.H.; Kwak, B.R.; Lacroix, J.S.; Huang, S.; et al. Cx26 regulates proliferation of repairing basal airway epithelial cells. Int. J. Biochem. Cell Biol. 2014, 52, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Razmovski-Naumovski, V.; Kota, B.P.; Lin, D.S.; Roufogalis, B.D. The pathophysiological function of peroxisome proliferator-activated receptor-γ in lung-related diseases. Respir. Res. 2005, 6, 102. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.F.; van der Ent, C.K.; Kalkhoven, E.; Beekman, J.M. PPARγ as a therapeutic target in cystic fibrosis. Trends Mol. Med. 2012, 18, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Bou Saab, J.; Bacchetta, M.; Chanson, M. Ineffective correction of PPARγ signaling in cystic fibrosis airway epithelial cells undergoing repair. Int. J. Biochem. Cell Biol. 2016, 78, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Bou Saab, J. Connexin26 and PPAR-Gamma Signaling Pathway in Human Airway Epithelial Cells. Ph.D. Thesis, University of Geneva, Geneva, Switzerland, 18 December 2015. [Google Scholar]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Horsley, V.; Fuchs, E. Epithelial stem cells: Turning over new leaves. Cell 2007, 128, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.L.; Patel, D.M.; Green, K.J. Deconstructing the skin: Cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Di, W.L.; Rugg, E.L.; Leigh, I.M.; Kelsell, D.P. Multiple epidermal connexins are expressed in different keratinocyte subpopulations including connexin 31. J. Investig. Dermatol. 2001, 117, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.E.; Easton, J.A.; Hodgins, M.B.; Wright, C.S. Connexins: Sensors of epidermal integrity that are therapeutic targets. FEBS Lett. 2014, 588, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Mesnil, M.; Krutovskikh, V.; Piccoli, C.; Elfgang, C.; Traub, O.; Willecke, K.; Yamasaki, H. Negative growth control of HeLa cells by connexin genes: Connexin species specificity. Cancer Res. 1995, 55, 629–639. [Google Scholar] [PubMed]
- Garcia, I.E.; Maripillan, J.; Jara, O.; Ceriani, R.; Palacios-Munoz, A.; Ramachandran, J.; Olivero, P.; Perez-Acle, T.; Gonzalez, C.; Saez, J.C.; et al. Keratitis-ichthyosis-deafness syndrome-associated cx26 mutants produce nonfunctional gap junctions but hyperactive hemichannels when co-expressed with wild type cx43. J. Investig. Dermatol. 2015, 135, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Easton, J.A.; Alboulshi, A.K.; Kamps, M.A.F.; Broers, G.H.M.R.; Coull, B.J.; Oji, V.; van Geel, M.; van Steensel, M.A.M.; Martin, P.E. A rare missense mutation in GJB3 (Cx31G45E) is associated with a unique cellular phenotype resulting in necrotic cell death. Exp. Dermatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kam, E.; Hodgins, M.B. Communication compartments in hair follicles and their implication in differentiative control. Development 1992, 114, 389–393. [Google Scholar] [PubMed]
- Lucke, T.; Choudhry, R.; Thom, R.; Selmer, I.S.; Burden, A.D.; Hodgins, M.B. Upregulation of connexin 26 is a feature of keratinocyte differentiation in hyperproliferative epidermis, vaginal epithelium, and buccal epithelium. J. Investig. Dermatol. 1999, 112, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.E.; van Steensel, M. Connexins and skin disease: Insights into the role of β connexins in skin homeostasis. Cell Tissue Res. 2015, 360, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Faniku, C.; Wright, C.S.; Martin, P.E. Connexins and pannexins in the integumentary system: The skin and appendages. Cell. Mol. Life Sci. 2015, 72, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Celetti, S.J.; Cowan, K.N.; Penuela, S.; Shao, Q.; Churko, J.; Laird, D.W. Implications of pannexin 1 and pannexin 3 for keratinocyte differentiation. J. Cell Sci. 2010, 123, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Penuela, S.; Celetti, S.J.; Bhalla, R.; Shao, Q.; Laird, D.W. Diverse subcellular distribution profiles of pannexin 1 and pannexin 3. Cell. Commun Adhes. 2008, 15, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Cowan, K.N.; Langlois, S.; Penuela, S.; Cowan, B.J.; Laird, D.W. Pannexin1 and Pannexin3 exhibit distinct localisation patterns in human skin appendages and are regulated during keratinocyte differentiation and carcinogenesis. Cell Commun. Adhes. 2012, 19, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, O.; Brem, H.; Vouthounis, C.; Lee, B.; Fallon, J.; Stallcup, M.; Merchant, A.; Galiano, R.D.; Tomic-Canic, M. Molecular pathogenesis of chronic wounds: The role of β-catenin and c-myc in the inhibition of epithelialization and wound healing. Am. J. Pathol. 2005, 167, 59–69. [Google Scholar] [CrossRef]
- Brandner, J.M.; Houdek, P.; Husing, B.; Kaiser, C.; Moll, I. Connexins 26, 30, and 43: Differences among spontaneous, chronic, and accelerated human wound healing. J. Investig. Dermatol. 2004, 122, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Solan, J.L.; Lampe, P.D. Kinase programs spatiotemporally regulate gap junction assembly and disassembly: Effects on wound repair. Semin. Cell Dev. Biol. 2016, 50, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Rosado, L.; Singh, D.; Rincon-Arano, H.; Solan, J.L.; Lampe, P.D. CASK (LIN2) interacts with Cx43 in wounded skin and their coexpression affects cell migration. J. Cell Sci. 2012, 125, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Faniku, C.; O’Shaughnessy, E.; Lorraine, C.; Johnstone, S.R.; Graham, A.; Greenhough, S.; Martin, P.E.M. The connexin mimetic peptide Gap27 and Cx43-knockdown reveal differential roles for Connexin43 in wound closure events in skin model systems. Int. J. Mol. Sci. 2018, 19, e604. [Google Scholar] [CrossRef] [PubMed]
- Hills, C.E.; Siamantouras, E.; Smith, S.W.; Cockwell, P.; Liu, K.K.; Squires, P.E. TGF-β modulates cell-to-cell communication in early epithelial-to-mesenchymal transition. Diabetologia 2012, 55, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Nakagami, T.; Tanaka, H.; Hitomi, T.; Takamatsu, T. Cx43 mediates TGF-β signaling through competitive Smads binding to microtubules. Mol. Biol Cell 2007, 18, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Kandyba, E.E.; Hodgins, M.B.; Martin, P.E. A murine living skin equivalent amenable to live-cell imaging: Analysis of the roles of connexins in the epidermis. J. Investig. Dermatol. 2008, 128, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Kretz, M.; Euwens, C.; Hombach, S.; Eckardt, D.; Teubner, B.; Traub, O.; Willecke, K.; Ott, T. Altered connexin expression and wound healing in the epidermis of connexin-deficient mice. J. Cell Sci. 2003, 116, 3443–3452. [Google Scholar] [CrossRef] [PubMed]
- Penuela, S.; Kelly, J.J.; Churko, J.M.; Barr, K.J.; Berger, A.C.; Laird, D.W. Panx1 regulates cellular properties of keratinocytes and dermal fibroblasts in skin development and wound healing. J. Investig. Dermatol. 2014, 134, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Game, F.L.; Apelqvist, J.; Attinger, C.; Hartemann, A.; Hinchliffe, R.J.; Londahl, M.; Price, P.E.; Jeffcoate, W.J. Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: A systematic review. Diabetes Metab. Res. Rev. 2016, 32, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.L.; Thrasivoulou, C.; Phillips, A.R. Connexins in wound healing; perspectives in diabetic patients. Biochim. Biophys. Acta 2012, 1818, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, J.E.; Chin, K.Y.; Thrasivoulou, C.; Serena, T.E.; O’Neil, S.; Hu, R.; White, A.M.; Madden, L.; Richards, T.; Phillips, A.R.; et al. Abnormal connexin expression in human chronic wounds. Br. J. Dermatol. 2015, 173, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.E. Connexins help fill the Gap: Markers and therapeutic targets for chronic nonhealing wounds. Br. J. Dermatol. 2015, 173, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Bowcock, A.M.; Krueger, J.G. Getting under the skin: The immunogenetics of psoriasis. Nat. Rev. Immunol. 2005, 5, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Buchau, A.S.; Gallo, R.L. Innate immunity and antimicrobial defense systems in psoriasis. Clin. Dermatol. 2007, 25, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.L.; Nakatsuji, T. Microbial symbiosis with the innate immune defense system of the skin. J. Investig. Dermatol. 2011, 131, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Enamandram, M.; Kimball, A.B. Psoriasis epidemiology: The interplay of genes and the environment. J. Investig. Dermatol. 2013, 133, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, N.; Poetzl, C.; von den Driesch, P.; Wladykowski, E.; Moll, I.; Behne, M.J.; Brandner, J.M. Alteration of tight junction proteins is an early event in psoriasis: Putative involvement of proinflammatory cytokines. Am. J. Pathol. 2009, 175, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S.; English, G.; de Zwart-Storm, E.A.; Lang, S.; van Steensel, M.A.; Martin, P.E. Differential susceptibility of Cx26 mutations associated with epidermal dysplasias to peptidoglycan derived from Staphylococcus aureus and Staphylococcus epidermidis. Exp. Dermatol. 2012, 21, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.P.; Stuart, P.E.; Nistor, I.; Hiremagalore, R.; Chia, N.V.; Jenisch, S.; Weichenthal, M.; Abecasis, G.R.; Lim, H.W.; Christophers, E.; et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am. J. Hum. Genet. 2006, 78, 827–851. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.D.; Cheng, H.; Wang, Z.X.; Zhang, A.P.; Wang, P.G.; Xu, J.H.; Zhu, Q.X.; Zhou, H.S.; Ellinghaus, E.; Zhang, F.R.; et al. Association analyses identify six new psoriasis susceptibility loci in the Chinese population. Nat. Genet. 2010, 42, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.P.; Wu, L.S.; Li, F.F.; Liu, S.; Su, J.; Kuang, Y.H.; Chen, C.; Xie, X.Y.; Jiang, M.H.; Zhao, S.; et al. The association between GJB2 gene polymorphism and psoriasis: A verification study. Arch. Dermatol. Res. 2012, 304, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ramirez, A.; Budunova, I. Overexpression of connexin26 in the basal keratinocytes reduces sensitivity to tumor promoter TPA. Exp. Dermatol. 2010, 19, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Yue, M.; Zhang, C.; Zuo, X.; Zheng, X.; Zhang, A.; Wang, Z.; Liu, S.; Li, H.; Meng, L.; et al. A genetic coding variant rs72474224 in GJB2 is associated with clinical features of psoriasis vulgaris in a Chinese Han population. Tissue Antigens 2015, 86, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tsoi, L.C.; Swindell, W.R.; Gudjonsson, J.E.; Tejasvi, T.; Johnston, A.; Ding, J.; Stuart, P.E.; Xing, X.; Kochkodan, J.J.; et al. Transcriptome analysis of psoriasis in a large case-control sample: RNA-seq provides insights into disease mechanisms. J. Investig. Dermatol. 2014, 134, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Labarthe, M.P.; Bosco, D.; Saurat, J.H.; Meda, P.; Salomon, D. Upregulation of connexin 26 between keratinocytes of psoriatic lesions. J. Investig. Dermatol. 1998, 111, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Shaker, O.; Abdel-Halim, M. Connexin 26 in psoriatic skin before and after two conventional therapeutic modalities: Methotrexate and PUVA. Eur. J. Dermatol. 2012, 22, 218–224. [Google Scholar] [PubMed]
- Moravcova, M.; Libra, A.; Dvorakova, J.; Viskova, A.; Muthny, T.; Velebny, V.; Kubala, L. Modulation of keratin 1, 10 and involucrin expression as part of the complex response of the human keratinocyte cell line HaCaT to ultraviolet radiation. Interdiscip. Toxicol. 2013, 6, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Djalilian, A.R.; McGaughey, D.; Patel, S.; Seo, E.Y.; Yang, C.; Cheng, J.; Tomic, M.; Sinha, S.; Ishida-Yamamoto, A.; Segre, J.A. Connexin 26 regulates epidermal barrier and wound remodeling and promotes psoriasiform response. J. Clin. Investig. 2006, 116, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Cook, P.W.; Parkos, C.A.; Park, Y.K.; Pittelkow, M.R.; Coffey, R.J. Amphiregulin causes functional downregulation of adherens junctions in psoriasis. J. Investig. Dermatol. 2005, 124, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Hampton, P.J.; Ross, O.K.; Reynolds, N.J. Increased nuclear β-catenin in suprabasal involved psoriatic epidermis. Br. J. Dermatol. 2007, 157, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, Z.; Wang, Y.; Geng, S.; Ji, F. Decreased expression of E-cadherin and β-catenin in the lesional skin of patients with active psoriasis. Int. J. Dermatol. 2008, 47, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Tschachler, E. ; Psoriasis: The epidermal component. Clin. Dermatol. 2007, 25, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Hivnor, C.; Williams, N.; Singh, F.; VanVoorhees, A.; Dzubow, L.; Baldwin, D.; Seykora, J. Gene expression profiling of porokeratosis demonstrates similarities with psoriasis. J. Cutan. Pathol. 2004, 31, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Acosta, N.; Whelan, F.J.; Somayaji, R.; Poonja, A.; Surette, M.G.; Rabin, H.R.; Parkins, M.D. The evolving cystic fibrosis microbiome: A comparative cohort study spanning 16 years. Ann. Am. Thorac. Soc. 2017, 14, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Castelino, M.; Eyre, S.; Upton, M.; Ho, P.; Barton, A. The bacterial skin microbiome in psoriatic arthritis, an unexplored link in pathogenesis: Challenges and opportunities offered by recent technological advances. Rheumatology 2014, 53, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.K.; Trolove, C.; Tattersall, D.; Thomas, A.C.; Papakonstantinopoulou, A.; Patel, D.; Scott, C.; Chong, J.; Jagger, D.J.; O’Toole, E.A.; et al. A deafness-associated mutant human connexin 26 improves the epithelial barrier in vitro. J. Membr. Biol. 2007, 218, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Losa, D.; Kohler, T.; Bacchetta, M.; Saab, J.B.; Frieden, M.; van Delden, C.; Chanson, M. Airway Epithelial cell integrity protects from cytotoxicity of pseudomonas aeruginosa quorum-sensing signals. Am. J. Respir. Cell Mol. Biol. 2015, 53, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Bou Saab, J.; Losa, D.; Chanson, M.; Ruez, R. Connexins in respiratory and gastrointestinal mucosal immunity. FEBS Lett. 2014, 588, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Braet, K.; Vandamme, W.; Martin, P.E.; Evans, W.H.; Leybaert, L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell. Calcium 2003, 33, 37–48. [Google Scholar] [CrossRef]
- De Vuyst, E.; Decrock, E.; de Bock, M.; Yamasaki, H.; Naus, C.C.; Evans, W.H.; Leybaert, L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol. Biol. Cell 2007, 18, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic signalling. Br. J. Pharmacol. 2006, 147, S172–S181. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.J.; Bowler, W.B.; Littlewood-Evans, A.; Dillon, J.P.; Bilbe, G.; Sharpe, G.R.; Gallagher, J.A. Regulation of epidermal homeostasis through P2Y2 receptors. Br. J. Pharmacol. 1999, 127, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Bikle, D.D. Adenosine triphosphate stimulates phosphoinositide metabolism, mobilizes intracellular calcium, and inhibits terminal differentiation of human epidermal keratinocytes. J. Clin. Investig. 1992, 90, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Inoue, K.; Fuziwara, S.; Denda, S. P2X purinergic receptor antagonist accelerates skin barrier repair and prevents epidermal hyperplasia induced by skin barrier disruption. J. Investig. Dermatol. 2002, 119, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Denda, S. Air-exposed keratinocytes exhibited intracellular calcium oscillation. Skin Res. Technol. 2007, 13, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ransford, G.A.; Fregien, N.; Qiu, F.; Dahl, G.; Conner, G.E.; Salathe, M. Pannexin 1 contributes to ATP release in airway epithelia. Am. J. Respir. Cell Mol. Biol. 2009, 41, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Seminario-Vidal, L.; Okada, S.F.; Sesma, J.I.; Kreda, S.M.; van Heusden, C.A.; Zhu, Y.; Jones, L.C.; O’Neal, W.K.; Penuela, S.; Laird, D.W.; et al. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J. Biol. Chem. 2011, 286, 26277–26286. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.H.; Bultynck, G.; Leybaert, L. Manipulating connexin communication channels: Use of peptidomimetics and the translational outputs. J. Membr. Biol. 2012, 245, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Kretz, M.; Maass, K.; Willecke, K. Expression and function of connexins in the epidermis, analyzed with transgenic mouse mutants. Eur. J. Cell Biol. 2004, 83, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Coutinho, P.; Frank, S.; Franke, S.; Law, L.Y.; Martin, P.; Green, C.R.; Becker, D.L. Targeting connexin43 expression accelerates the rate of wound repair. Curr. Biol. 2003, 13, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Lincoln, J.; Cook, J.E.; Becker, D.L. Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes 2007, 56, 2809–2817. [Google Scholar] [CrossRef] [PubMed]
- Law, L.Y.; Zhang, W.V.; Stott, N.S.; Becker, D.L.; Green, C.R. In vitro optimization of antisense oligodeoxynucleotide design: An example using the connexin gene family. J. Biomol. Tech. 2006, 17, 270–282. [Google Scholar] [PubMed]
- Mori, R.; Power, K.T.; Wang, C.M.; Martin, P.; Becker, D.L. Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J. Cell Sci. 2006, 119, 5193–5203. [Google Scholar] [CrossRef] [PubMed]
- Cronin, M.; Anderson, P.N.; Cook, J.E.; Green, C.R.; Becker, D.L. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol. Cell. Neurosci. 2008, 39, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Meyer, H.V.; Huang, R.; Nicholson, L.F.; Green, C.R. Connexin43 antisense oligodeoxynucleotide treatment down-regulates the inflammatory response in an in vitro interphase organotypic culture model of optic nerve ischaemia. J. Clin. Neurosci. 2008, 15, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Green, C.R.; Nicholson, L.F. Interrupting the inflammatory cycle in chronic diseases- do gap junctions provide the answer? Cell Biol. Int. 2008, 32, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Ghatnekar, G.S.; Grek, C.L.; Armstrong, D.G.; Desai, S.C.; Gourdie, R.G. The effect of a connexin43-based Peptide on the healing of chronic venous leg ulcers: A multicenter, randomized trial. J. Investig. Dermatol. 2015, 135, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ghatnekar, G.S.; O’Quinn, M.P.; Jourdan, L.J.; Gurjarpadhye, A.A.; Draughn, R.L.; Gourdie, R.G. Connexin43 carboxyl-terminal peptides reduce scar progenitor and promote regenerative healing following skin wounding. RegenMed 2009, 4, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Ongstad, E.L.; O’Quinn, M.P.; Ghatnekar, G.S.; Yost, M.J.; Gourdie, R.G. A Connexin43 Mimetic Peptide Promotes Regenerative Healing and Improves Mechanical Properties in Skin and Heart. Adv. Wound Care 2013, 2, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Rhett, J.M.; Ghatnekar, G.S.; Palatinus, J.A.; O’Quinn, M.; Yost, M.J.; Gourdie, R.G. Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol. 2008, 26, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Berends, R.F.; Flint, D.J.; Martin, P.E. Cell motility in models of wounded human skin is improved by Gap27 despite raised glucose, insulin and IGFBP-5. Exp. Cell Res. 2013, 319, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Pollok, S.; Flint, D.J.; Brandner, J.M.; Martin, P.E. The connexin mimetic peptide Gap27 increases human dermal fibroblast migration in hyperglycemic and hyperinsulinemic conditions in vitro. J. Cell. Physiol. 2012, 227, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; van Steensel, M.A.; Hodgins, M.B.; Martin, P.E. Connexin mimetic peptides improve cell migration rates of human epidermal keratinocytes and dermal fibroblasts in vitro. Wound Repair Regen. 2009, 17, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Riding, A.; Pullar, C.E. ATP Release and P2 Y Receptor signaling are essential for keratinocyte galvanotaxis. J. Cell. Physiol. 2016, 231, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Riteau, N.; Gasse, P.; Fauconnier, L.; Gombault, A.; Couegnat, M.; Fick, L.; Kanellopoulos, J.; Quesniaux, V.F.; Marchand-Adam, S.; Crestani, B.; et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2011, 182, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.R.; Joung, J.K. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 227–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urasaki, A.; Morvan, G.; Kawakami, K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics 2006, 174, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Frank, M.; Thisse, C.I.; Thisse, B.V.; Uitto, J. Zebrafish: A model system to study heritable skin diseases. J. Investig. Dermatol. 2011, 131, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Uitto, J. Zebrafish as a model system to study skin biology and pathology. J. Investig. Dermatol. 2014, 134, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M. Gap junction in the teleost fish lineage: duplicated connexins may contribute to skin pattern formation and body shape determination. Front. Cell Dev. Biol. 2017, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Chin, A.J.; Valdimarsson, G.; Finis, C.; Sonntag, J.M.; Choi, B.Y.; Tao, L.; Balasubramanian, K.; Bell, C.; Krufka, A.; et al. Developmental regulation and expression of the zebrafish connexin43 gene. Dev. Dyn. 2005, 233, 890–906. [Google Scholar] [CrossRef] [PubMed]
- Iovine, M.K.; Higgins, E.P.; Hindes, A.; Coblitz, B.; Johnson, S.L. Mutations in connexin43 (GJA1) perturb bone growth in zebrafish fins. Dev. Biol. 2005, 278, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Irion, U.; Frohnhofer, H.G.; Krauss, J.; Colak Champollion, T.; Maischein, H.M.; Geiger-Rudolph, S.; Weiler, C.; Nusslein-Volhard, C. Gap junctions composed of connexins 41.8 and 39.4 are essential for colour pattern formation in zebrafish. eLife 2014, 3, e05125. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Sawada, R.; Aramaki, T.; Skerrett, I.M.; Kondo, S. The physiological characterization of connexin41.8 and connexin39.4, which are involved in the striped pattern formation of zebrafish. J. Biol. Chem. 2016, 291, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Nakada, Y.; Canseco, D.C.; Thet, S.; Abdisalaam, S.; Asaithamby, A.; Santos, C.X.; Shah, A.M.; Zhang, H.; Faber, J.E.; Kinter, M.T.; et al. Hypoxia induces heart regeneration in adult mice. Nature 2017, 541, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Sehring, I.M.; Jahn, C.; Weidinger, G. Zebrafish fin and heart: What’s special about regeneration? Curr. Opin. Genet. Dev. 2016, 40, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Shakespeare, T.; Mui, R.; White, T.W.; Valdimarsson, G. Connexin 48.5 is required for normal cardiovascular function and lens development in zebrafish embryos. J. Biol. Chem. 2004, 279, 36993–37003. [Google Scholar] [CrossRef] [PubMed]
- Christie, T.L.; Mui, R.; White, T.W.; Valdimarsson, G. Molecular cloning, functional analysis, and RNA expression analysis of connexin45.6: A zebrafish cardiovascular connexin. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1623–H1632. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Nag, K.; Hoshijima, K.; Laird, D.W.; Kawakami, A.; Hirose, S. Zebrafish early cardiac connexin, Cx36.7/Ecx, regulates myofibril orientation and heart morphogenesis by establishing Nkx2.5 expression. Proc. Natl. Acad. Sci. USA 2008, 105, 4763–4768. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Iwashita, M.; Ishii, M.; Kurachi, Y.; Kawakami, A.; Kondo, S.; Okada, N. Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene. EMBO Rep. 2006, 7, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Sallin, P.; de Preux Charles, A.S.; Duruz, V.; Pfefferli, C.; Jazwinska, A. A dual epimorphic and compensatory mode of heart regeneration in zebrafish. Dev. Biol. 2015, 399, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, R.; Parks, E.; Takabe, W.; Hsiai, T.K. Electrocardiogram signals to assess zebrafish heart regeneration: Implication of long QT intervals. Ann. Biomed. Eng. 2010, 38, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Hoptak-Solga, A.D.; Klein, K.A.; DeRosa, A.M.; White, T.W.; Iovine, M.K. Zebrafish short fin mutations in connexin43 lead to aberrant gap junctional intercellular communication. FEBS Lett. 2007, 581, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
- Sims, K., Jr.; Eble, D.M.; Iovine, M.K. Connexin43 regulates joint location in zebrafish fins. Dev. Biol. 2009, 327, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Fisher, S.; Iovine, M.K. Osteoblast maturation occurs in overlapping proximal-distal compartments during fin regeneration in zebrafish. Dev. Dyn. 2009, 238, 2922–2928. [Google Scholar] [CrossRef] [PubMed]
- Hoptak-Solga, A.D.; Nielsen, S.; Jain, I.; Thummel, R.; Hyde, D.R.; Iovine, M.K. Connexin43 (GJA1) is required in the population of dividing cells during fin regeneration. Dev. Biol. 2008, 317, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Ton, Q.V.; Iovine, M.K. Identification of an evx1-dependent joint-formation pathway during FIN regeneration. PLoS ONE 2013, 8, e81240. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.J.; Allen, C.; England, S. J.; Juarez-Morales, J.L.; Lewis, K.E. Evx1 is required for joint formation in zebrafish fin dermoskeleton. Dev. Dyn. 2011, 240, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Dardis, G.; Tryon, R.; Ton, Q.; Johnson, S.L.; Iovine, M.K. Cx43 suppresses evx1 expression to regulate joint initiation in the regenerating fin. Dev. Dyn. 2017, 246, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Ton, Q.V.; Kathryn Iovine, M. Semaphorin3d mediates Cx43-dependent phenotypes during fin regeneration. Dev. Biol. 2012, 366, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Govindan, J.; Iovine, M.K. Hapln1a is required for connexin43-dependent growth and patterning in the regenerating fin skeleton. PLoS ONE 2014, 9, e88574. [Google Scholar] [CrossRef] [PubMed]
- Govindan, J.; Tun, K.M.; Iovine, M.K. Cx43-dependent skeletal phenotypes are mediated by interactions between the Hapln1a-ECM and Sema3d during fin regeneration. PLoS ONE 2016, 11, e0148202. [Google Scholar] [CrossRef] [PubMed]
- Gerhart, S.V.; Eble, D.M.; Burger, R.M.; Oline, S.N.; Vacaru, A.; Sadler, K.C.; Jefferis, R.; Iovine, M.K. The Cx43-like connexin protein Cx40.8 is differentially localized during fin ontogeny and fin regeneration. PLoS ONE 2012, 7, e31364. [Google Scholar] [CrossRef] [PubMed]
- Gerhart, S.V.; Jefferis, R.; Iovine, M.K. Cx40.8, a Cx43-like protein, forms gap junction channels inefficiently and may require Cx43 for its association at the plasma membrane. FEBS Lett. 2009, 583, 3419–3424. [Google Scholar] [CrossRef] [PubMed]
- Grupcheva, C.N.; Laux, W.T.; Rupenthal, I.D.; McGhee, J.; McGhee, C.N.; Green, C.R. Improved corneal wound healing through modulation of gap junction communication using connexin43-specific antisense oligodeoxynucleotides. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Bryant, Z.J.; Ghatnekar, G.; Singh, U.P.; Gourdie, R.G.; Potts, J.D. A synthetic connexin 43 mimetic peptide augments corneal wound healing. Exp. Eye Res. 2013, 115, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Ormonde, S.; Chou, C.Y.; Goold, L.; Petsoglou, C.; Al-Taie, R.; Sherwin, T.; McGhee, C.N.; Green, C.R. Regulation of connexin43 gap junction protein triggers vascular recovery and healing in human ocular persistent epithelial defect wounds. J. Membr. Biol. 2012, 245, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Elbadawy, H.M.; Mirabelli, P.; Xeroudaki, M.; Parekh, M.; Bertolin, M.; Breda, C.; Cagini, C.; Ponzin, D.; Lagali, N.; Ferrari, S. Effect of connexin 43 inhibition by the mimetic peptide Gap27 on corneal wound healing, inflammation and neovascularization. Br. J. Pharmacol. 2016, 173, 2880–2893. [Google Scholar] [CrossRef] [PubMed]
- Bellec, J.; Bacchetta, M.; Losa, D.; Anegon, I.; Chanson, M.; Nguyen, T.H. CFTR inactivation by lentiviral vector-mediated RNA interference and CRISPR-Cas9 genome editing in human airway epithelial cells. Curr. Gene Ther. 2015, 15, 447–459. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanson, M.; Watanabe, M.; O’Shaughnessy, E.M.; Zoso, A.; Martin, P.E. Connexin Communication Compartments and Wound Repair in Epithelial Tissue. Int. J. Mol. Sci. 2018, 19, 1354. https://doi.org/10.3390/ijms19051354
Chanson M, Watanabe M, O’Shaughnessy EM, Zoso A, Martin PE. Connexin Communication Compartments and Wound Repair in Epithelial Tissue. International Journal of Molecular Sciences. 2018; 19(5):1354. https://doi.org/10.3390/ijms19051354
Chicago/Turabian StyleChanson, Marc, Masakatsu Watanabe, Erin M. O’Shaughnessy, Alice Zoso, and Patricia E. Martin. 2018. "Connexin Communication Compartments and Wound Repair in Epithelial Tissue" International Journal of Molecular Sciences 19, no. 5: 1354. https://doi.org/10.3390/ijms19051354
APA StyleChanson, M., Watanabe, M., O’Shaughnessy, E. M., Zoso, A., & Martin, P. E. (2018). Connexin Communication Compartments and Wound Repair in Epithelial Tissue. International Journal of Molecular Sciences, 19(5), 1354. https://doi.org/10.3390/ijms19051354