A Novel Action of Endocrine-Disrupting Chemicals on Wildlife; DDT and Its Derivatives Have Remained in the Environment
Abstract
:1. Introduction
2. Continuing Obscurity of the Molecular Mechanisms of Adverse Effects in Alligators in Lake Apopka
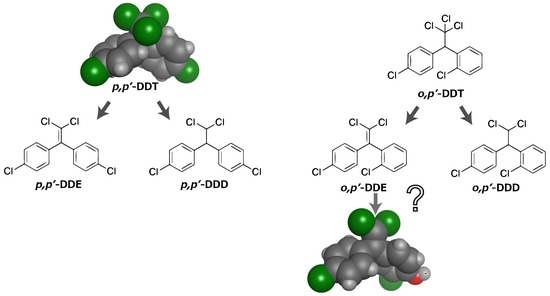
3. Dichlorodiphenyltrichloroethane (DDT) Metabolites and Their Structurally Analogous Chemical Compounds Bisphenol C (BPC)
4. Halogen Bonds in Biological Molecules
5. The Possibility of the Conversion from DDT to BPC
6. Conclusions and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| BPA | Bisphenol A |
| BPC | Bisphenol C |
| EDC | Endocrine-disrupting chemical |
| DDT | Dichlorodiphenyltrichloroethane |
| DDE | Dichlorodiphenyldichloroethylene |
| ER | Estrogen receptor |
| HTPE | 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane |
References
- Woodward, A.R.; Percival, H.F.; Jennings, M.L.; Moore, C.T. Low clutch viability of american alligators on lake apopka. Fla. Sci. 1993, 56, 52–63. [Google Scholar]
- Kelce, W.R.; Stone, C.R.; Laws, S.C.; Gray, L.E.; Kemppainen, J.A.; Wilson, E.M. Persistent ddt metabolite p,p’-dde is a potent androgen receptor antagonist. Nature 1995, 375, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Methoxychlor (content source: Agency for Toxic Substances and Disease Registry). Available online: https://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=151 (accessed on 5 May 2018).
- Matsushima, A.; Liu, X.; Okada, H.; Shimohigashi, M.; Shimohigashi, Y. Bisphenol af is a full agonist for the estrogen receptor eralpha but a highly specific antagonist for erbeta. Environ. Health Perspect. 2010, 118, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Gaido, K.W.; Leonard, L.S.; Maness, S.C.; Hall, J.M.; McDonnell, D.P.; Saville, B.; Safe, S. Differential interaction of the methoxychlor metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane with estrogen receptors α and β. Endocrinology 1999, 140, 5746–5753. [Google Scholar] [CrossRef] [PubMed]
- Guillette, L.J., Jr.; Gross, T.S.; Masson, G.R.; Matter, J.M.; Percival, H.F.; Woodward, A.R. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in florida. Environ. Health Perspect. 1994, 102, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Guillette, L.J., Jr.; Crain, D.A.; Rooney, A.A.; Pickford, D.B. Organization versus activation: The role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environ. Health Perspect. 1995, 103, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Guillette, L.J.; Gross, T.S.; Gross, D.A.; Rooney, A.A.; Percival, H.F. Gonadal steroidogenesis in vitro from juvenile alligators obtained from contaminated or control lakes. Environ. Health Perspect. 1995, 103, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Guillette, L.J.; Pickford, D.B.; Crain, D.A.; Rooney, A.A.; Percival, H.F. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen. Comp. Endocrinol. 1996, 101, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S.; Bermudez, D.S.; Katsu, Y.; Iguchi, T.; Guillette, L.J. Gene expression patterns in juvenile American alligators (Alligator Mississippiensis) exposed to environmental contaminants. Aquat. Toxicol. 2008, 88, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Milnes, M.R.; Bryan, T.A.; Katsu, Y.; Kohno, S.; Moore, B.C.; Iguchi, T.; Guillette, L.J. Increased posthatching mortality and loss of sexually dimorphic gene expression in alligators (Alligator Mississippiensis) from a contaminated environment. Biol. Reprod. 2008, 78, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Vonier, P.M.; Crain, D.A.; McLachlan, J.A.; Guillette, L.J.; Arnold, S.F. Interaction of environmental chemicals with the estrogen and progesterone receptors from the oviduct of the American alligator. Environ. Health Perspect. 1996, 104, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Heinz, G.H.; Percival, H.F.; Jennings, M.L. Contaminants in american alligator eggs from Lake Apopka, Lake Griffin, and Lake Okeechobee, Florida. Environ. Monit. Assess. 1991, 16, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Milnes, M.R.; Woodward, A.R.; Rooney, A.A.; Guillette, L.J. Plasma steroid concentrations in relation to size and age in juvenile alligators from two Florida lakes. Comp. Biochem. Physiol. 2002, 131, 923–930. [Google Scholar] [CrossRef]
- Gunderson, M.P.; Bermudez, D.S.; Bryan, T.A.; Degala, S.; Edwards, T.M.; Kools, S.A.E.; Milnes, M.R.; Woodward, A.R.; Guillette, L.J. Variation in sex steroids and phallus size in juvenile American alligators (Alligator Mississippiensis) collected from 3 sites within the kissimmee-everglades drainage in Florida (USA). Chemosphere 2004, 56, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Guillette, L.J.; Woodward, A.R.; Crain, D.A.; Pickford, D.B.; Rooney, A.A.; Percival, H.F. Plasma steroid concentrations and male phallus size in juvenile alligators from seven florida lakes. Gen. Comp. Endocrinol. 1999, 116, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Guillette, L.J., Jr.; Brock, J.W.; Rooney, A.A.; Woodward, A.R. Serum concentrations of various environmental contaminants and their relationship to sex steroid concentrations and phallus size in juvenile American alligators. Arch. Environ. Contam. Toxicol. 1999, 36, 447–455. [Google Scholar] [CrossRef] [PubMed]
- St John, J.A.; Braun, E.L.; Isberg, S.R.; Miles, L.G.; Chong, A.Y.; Gongora, J.; Dalzell, P.; Moran, C.; Bed’Hom, B.; Abzhanov, A.; et al. Sequencing three crocodilian genomes to illuminate the evolution of archosaurs and amniotes. Gen. Biol. 2012, 13, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yatsu, R.; Miyagawa, S.; Kohno, S.; Parrott, B.B.; Yamaguchi, K.; Ogino, Y.; Miyakawa, H.; Lowers, R.H.; Shigenobu, S.; Guillette, L.J.; et al. RNA-seq analysis of the gonadal transcriptome during Alligator Mississippiensis temperature-dependent sex determination and differentiation. BMC Genom. 2016, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- DDT and Its Derivatives. Available online: http://www.inchem.org/documents/ehc/ehc/ehc009.htm (accessed on 5 May 2018).
- DDT and Its Derivatives-Environmantal Aspects. Available online: http://www.inchem.org/documents/ehc/ehc/ehc83.htm (accessed on 5 May 2018).
- Ricking, M.; Schwarzbauer, J. Ddt isomers and metabolites in the environment: An overview. Environ. Chem. Lett. 2012, 10, 317–323. [Google Scholar] [CrossRef]
- Turusov, V.; Rakitsky, V.; Tomatis, L. Dichlorodiphenyltrichloroethane (DDT): Ubiquity, persistence, and risks. Environ. Health Perspect. 2002, 110, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Shimizu, Y.; Shiraga, Y.; Yoshida, M.; Sugihara, K.; Ohta, S. Reductive metabolism of p,p′-DDT and o,p′-DDT by rat liver cytochrome p450. Drug Metab. Disposit. 2002, 30, 113–118. [Google Scholar] [CrossRef]
- Kirman, C.R.; Aylward, L.L.; Hays, S.M.; Krishnan, K.; Nong, A. Biomonitoring equivalents for DDT/DDE. Regul. Toxicol. Pharmacol. 2011, 60, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Van der Oost, R.; Beyer, J.; Vermeulen, N. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Colborn, T.; vom Saal, F.S.; Soto, A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yin, S.; Tang, M.; Liu, K.; Yang, F.; Liu, W. Environmental exposure to DDT and its metabolites in cord serum: Distribution, enantiomeric patterns, and effects on infant birth outcomes. Sci. Total Environ. 2017, 580, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.B.; Curtis, L.R.; Williams, D.E. Salmonid sexual development is not consistently altered by embryonic exposure to endocrine-active chemicals. Environ. Health Perspect. 2000, 108, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, C.; Peng, H.; Zheng, G.; Zhang, S.; Hu, J. p,p’-DDE induces gonadal intersex in japanese medaka (Oryzias Latipes) at environmentally relevant concentrations: Comparison with o,p-DDT. Environ. Sci. Technol. 2016, 50, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Snedeker, S.M. Pesticides and breast cancer risk: A review of DDT, DDE, and dieldrin. Environ. Health Perspect. 2001, 109, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Howe, S.R.; Borodinsky, L. Potential exposure to Bisphenol A from food-contact use of polycarbonate resins. Food Addit. Contam. 1998, 15, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Dorn, P.B.; Klecka, G.M.; O’Block, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Lewis, J.B.; Rueggeberg, F.A.; Lapp, C.A.; Ergle, J.W.; Schuster, G.S. Identification and characterization of estrogen-like components in commercial resin-based dental restorative materials. Clin. Oral Investig. 1999, 3, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Crain, D.A.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; LeBlanc, G.A.; Guillette, L.J. An ecological assessment of Bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Kannan, K. Concentrations and profiles of Bisphenol A and other bisphenol analogues in foodstuffs from the united states and their implications for human exposure. J. Agric. Food Chem. 2013, 61, 4655–4662. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, A.; Ryan, K.; Shimohigashi, Y.; Meinertzhagen, I.A. An endocrine disruptor, Bisphenol A, affects development in the protochordate ciona intestinalis: Hatching rates and swimming behavior alter in a dose-dependent manner. Environ. Pollut. 2013, 173, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.; Williams, P.L.; Missmer, S.A.; Flaws, J.A.; Ye, X.; Calafat, A.M.; Petrozza, J.C.; Wright, D.; Hauser, R. Urinary Bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum. Reprod. 2012, 27, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- Völkel, W.; Kiranoglu, M.; Fromme, H. Determination of free and total Bisphenol A in urine of infants. Environ. Res. 2011, 111, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Welshons, W.V. Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that bpa causes numerous hazards from multiple routes of exposure. Mol. Cell. Endocrinol. 2014, 398, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Shelby, M.D. NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Bisphenol A. 2008. Available online: https://ntp.niehs.nih.gov/ntp/ohat/bisphenol/bisphenol.pdf (accessed on 5 May 2018).
- Shelnutt, S.; Kind, J.; Allaben, W. Bisphenol A: Update on newly developed data and how they address NTP’s 2008 finding of “some concern”. Food Chem. Toxicol. 2013, 57, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Grob, K.; Gürtler, R.; Husøy, T.; Mennes, W.; Milana, M.R. Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Dodds, E.C.; Lawson, W. Synthetic estrogenic agents without the phenanthrene nucleus. Nature 1936, 137, 996. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Stathis, P.; Permuth, S.F.; Tokes, L.; Feldman, D. Bisphenol-A: An estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 1993, 132, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Olea, N.; Pulgar, R.; Pérez, P.; Olea-Serrano, F.; Rivas, A.; Novillo-Fertrell, A.; Pedraza, V.; Soto, A.M.; Sonnenschein, C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ. Health Perspect. 1996, 104, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.M.; Gore, A.C. Transgenerational neuroendocrine disruption of reproduction. Nat. Rev. Endocrinol. 2011, 7, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.-H.; Pan, J.-X.; Jin, L.-Y.; Xu, H.-Y.; Yu, T.-T.; Ullah, K.; Rahman, T.U.; Ren, J.; Cheng, Y.; Dong, X.-Y.; et al. Bisphenol A exposure may induce hepatic lipid accumulation via reprogramming the DNA methylation patterns of genes involved in lipid metabolism. Sci. Rep. 2016, 6, 31331. [Google Scholar] [CrossRef] [PubMed]
- León-Olea, M.; Martyniuk, C.J.; Orlando, E.F.; Ottinger, M.A.; Rosenfeld, C.S.; Wolstenholme, J.T.; Trudeau, V.L. Current concepts in neuroendocrine disruption. Gen. Comp. Endcrinol. 2014, 203, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Casati, L.; Sendra, R.; Sibilia, V.; Celotti, F. Endocrine disrupters: The new players able to affect the epigenome. Front. Cell Dev. Biol. 2015, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Shafei, A.; Ramzy, M.M.; Hegazy, A.I.; Husseny, A.K.; EL-hadary, U.G.; Taha, M.M.; Mosa, A.A. The molecular mechanisms of action of the endocrine disrupting chemical Bisphenol A in the development of cancer. Gene 2018, 647, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Nagel, S.C.; vom Saal, F.S.; Thayer, K.A.; Dhar, M.G.; Boechler, M.; Welshons, W.V. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens Bisphenol A and octylphenol. Environ. Health Perspect. 1997, 105, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc. Soc. Exp. Biol. Med. 2000, 224, 61–68. [Google Scholar] [CrossRef] [PubMed]
- vom Saal, F.S.; Hughes, C. An extensive new literature concerning low-dose effects of Bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005, 113, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, J.T.; Edwards, M.; Shetty, S.R.J.; Gatewood, J.D.; Taylor, J.A.; Rissman, E.F.; Connelly, J.J. Gestational exposure to Bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology 2012, 153, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, S.; Tokunaga, T.; Liu, X.; Okada, H.; Matsushima, A.; Shimohigashi, Y. Endocrine disruptor Bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity. Toxicol. Lett. 2006, 167, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, A.; Kakuta, Y.; Teramoto, T.; Koshiba, T.; Liu, X.; Okada, H.; Tokunaga, T.; Kawabata, S.-I.; Kimura, M.; Shimohigashi, Y. Structural evidence for endocrine disruptor Bisphenol A binding to human nuclear receptor ERRγ. J. Biochem. 2007, 142, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, A.; Teramoto, T.; Okada, H.; Liu, X.; Tokunaga, T.; Kakuta, Y.; Shimohigashi, Y. Errgamma tethers strongly Bisphenol A and 4-α-cumylphenol in an induced-fit manner. Biochem. Biophys. Res. Commun. 2008, 373, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Matsushima, A.; Okada, H.; Shimohigashi, Y. Distinction of the binding modes for human nuclear receptor ERRγ between bisphenol A and 4-hydroxytamoxifen. J. Biochem. 2010, 148, 247–254. [Google Scholar] [CrossRef] [PubMed]
- DeKeyser, J.G.; Laurenzana, E.M.; Peterson, E.C.; Chen, T.; Omiecinski, C.J. Selective phthalate activation of naturally occurring human constitutive androstane receptor splice variants and the pregnane X receptor. Toxicol. Sci. 2011, 120, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.S.; Bucher, J.R.; Collman, G.W.; Zeldin, D.C.; Johnson, A.F.; Schug, T.T.; Heindel, J.J. Consortium-based science: The NIEHS’s multipronged, collaborative approach to assessing the health effects of bisphenol A. Environ. Health Perspect. 2012, 120, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Newbold, R.R.; Bucher, J.R.; Camacho, L.; Delclos, K.B.; Lewis, S.M.; Vanlandingham, M.; Churchwell, M.I.; Twaddle, N.C.; McLellen, M.; et al. NIEHS/FDA CLARITY-BPA research program update. Reprod. Toxicol. 2015, 58, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Neuroendocrine disruption in animal models due to exposure to Bisphenol A analogues. Front. Neuroendocrinol. 2017, 47, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Porejko, S.; Brzozowski, Z.K.; Maczynski, C.; Wielgosz, Z. Process for synthesizing self-extinguishing thermoplastics. Polish Patent 48,893, 12 December 1964. [Google Scholar]
- Porejko, S.; Wielgosz, Z. Synthesis and properties of polycarbonates with chlorobisphenols. Polimery 1968, 13, 55. [Google Scholar]
- Dobkowski, Z.; Grzelak, D. Specific volume of bisphenol C-2 polycarbonate. Eur. Polym. J. 1984, 20, 1045–1047. [Google Scholar] [CrossRef]
- Factor, A.; Orlando, C.M. Polycarbonates from 1,1-dichloro-2,2-bis(4-hydroxyphenyl)ethylene and Bisphenol A: A highly flame-resistant family of engineering thermoplastics. J. Polym. Sci. 1980, 18, 579–592. [Google Scholar]
- Jurs, J.L.; Tour, J.M. Novel flame retardant polyarylethers: Synthesis and testing. Polymer 2003, 44, 3709–3714. [Google Scholar] [CrossRef]
- Ellzey, K.A.; Farris, R.J.; Emrick, T. Synthetic and thermal studies of bisphenol-C containing poly(aryletherketone). Polym. Bull. 2003, 50, 235–242. [Google Scholar]
- Stoliarov, S.I.; Westmoreland, P.R. Mechanism of the thermal decomposition of bisphenol C polycarbonate: Nature of its fire resistance. Polymer 2003, 44, 5469–5475. [Google Scholar] [CrossRef]
- Deceuninck, Y.; Bichon, E.; Marchand, P. Determination of Bisphenol A and related substitutes/analogues in human breast milk using gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2485–2497. [Google Scholar] [CrossRef] [PubMed]
- Česen, M.; Levstek, M.; Cimrmančič, B.; Heath, D.; Dolenc, M.S. The occurrence and source identification of bisphenol compounds in wastewaters. Sci. Total Environ. 2018, 616–617, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shan, G.; Zhu, L. Bioaccumulation and biomagnification of emerging bisphenol analogues in aquatic organisms from taihu lake, China. Sci. Total Environ. 2017, 598, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Delfosse, V.; Grimaldi, M.; Pons, J.L.; Boulahtouf, A.; Le Maire, A.; Cavaillès, V.; Labesse, G.; Bourguet, W.; Balaguer, P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of Bisphenol A substitutes. Proc. Natl. Acad. Sci. USA 2012, 109, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
- Cavaillès, V. Structural and functional profiling of environmental ligands for estrogen receptors. Environ. Health Perspect. 2015, 122, 1306–1313. [Google Scholar]
- Cui, S.; Liu, S.; Yang, J.; Wang, X.; Wang, L. Quantitative structure-activity relationship of estrogen activities of Bisphenol A analogs. Chin. Sci. Bull. 2006, 51, 287–292. [Google Scholar] [CrossRef]
- Yang, X.; Liu, H.; Yang, Q. Predicting anti-androgenic activity of bisphenols using molecular docking and quantitative structure-activity relationships. Chemosphere 2016, 163, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, H.; Takeda, S.; Kakizoe, K.; Taniguchi, A.; Tokuyasu, M.; Himeno, T.; Ishii, H.; Kohro-Ikeda, E.; Haraguchi, K.; Watanabe, K.; et al. Bisphenol af as an inducer of estrogen receptor β (ERβ): Evidence for anti-estrogenic effects at higher concentrations in human breast cancer cells. Biol. Pharm. Bull. 2017, 40, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Akingbemi, B.T.; Ge, R.S.; Klinefelter, G.R.; Gunsalus, G.L.; Hardy, M.P. A metabolite of methoxychlor, 2,2-bis(p-hydroxyphenyl)-1,1, 1-trichloroethane, reduces testosterone biosynthesis in rat leydig cells through suppression of steady-state messenger ribonucleic acid levels of the cholesterol side-chain cleavage enzyme. Biol. Reprod. 2000, 62, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Waters, K.M.; Safe, S.; Gaido, K.W. Differential gene expression in response to methoxychlor and estradiol through er alpha, er beta, and ar in reproductive tissues of female mice. Toxicol. Sci. 2001, 63, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.C.; Korach, K.S. Estrogenic activity of Bisphenol A and 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (HTPE) demonstrated in mouse uterine gene profiles. Environ. Health Perspect. 2011, 119, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.N.; Esmail, M.; Wang, Q.; Brooks, A.I.; Zachow, R.; Uzumcu, M. Effect of the methoxychlor metabolite hpte on the rat ovarian granulosa cell transcriptome in vitro. Toxicol. Sci. 2009, 110, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.N.; Chen, J.C.; Bagnell, C.A.; Uzumcu, M. Methoxychlor and its metabolite hpte inhibit camp production and expression of estrogen receptors α and β in the rat granulosa cell in vitro. Reprod. Toxicol. 2015, 51, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Akgul, Y.; Derk, R.C.; Meighan, T.; Rao, K.M.K.; Murono, E.P. The methoxychlor metabolite, hpte, directly inhibits the catalytic activity of cholesterol side-chain cleavage (p450scc) in cultured rat ovarian cells. Reprod. Toxicol. 2008, 25, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chen, X.; Li, X.; Zhu, Q.; Yu, L.; Guo, J.; Chen, B.; Akingbemi, B.T.; Ge, R.-S.; Li, H. Effects of methoxychlor and its metabolite 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane on human and rat 17α-hydroxylase/17,20-lyase activity. Toxicol. Lett. 2014, 225, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.X.; Zhao, B.; Chu, Y.; Li, X.H.; Akingbemi, B.T.; Zheng, Z.Q.; Ge, R.S. Effects of methoxychlor and 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane on 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase-3 activities in human and rat testes. Int. J. Androl. 2011, 34, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, S.; Safe, S. Activation of kinase pathways in MCF-7 cells by 17beta-estradiol and structurally diverse estrogenic compounds. J. Steroid Biochem. Mol. Biol. 2006, 98, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Manna, D.; Mugesh, G. Regioselective deiodination of thyroxine by iodothyronine deiodinase mimics: An unusual mechanistic pathway involving cooperative chalcogen and halogen bonding. J. Am. Chem. Soc. 2012, 134, 4269–4279. [Google Scholar] [CrossRef] [PubMed]
- Rigét, F.; Vorkamp, K.; Bossi, R.; Sonne, C.; Letcher, R.J.; Dietz, R. Twenty years of monitoring of persistent organic pollutants in greenland biota. A review. Environ. Pollut. 2016, 217, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Jepson, P.D.; Science, R.L. Persistent pollutants, persistent threats. Science 2016, 352, 1388–1389. [Google Scholar] [CrossRef] [PubMed]
- Srogi, K. Levels and congener distributions of pcdds, pcdfs and dioxin-like pcbs in environmental and human samples: A review. Environ. Chem. Lett. 2008, 6, 1–28. [Google Scholar] [CrossRef]
- Safe, S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: Environmental and mechanistic considerations which support the development of toxic equivalancy Factors (TEFs). Crit. Rev. Toxicol. 2008, 21, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Kolář, M.H.; Hobza, P. Computer modeling of halogen bonds and other σ-hole interactions. Chem. Rev. 2016, 116, 5155–5187. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. The bright future of unconventional σ/π-hole interactions. ChemPhysChem 2015, 16, 2496–2517. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, P.; Hays, F.A.; Westhof, E.; Ho, P.S. Halogen bonds in biological molecules. Proc. Natl. Acad. Sci. USA 2004, 101, 16789–16794. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C. Definition of the halogen bond (IUPAC recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Persch, E.; Dumele, O.; Diederich, F. Molecular recognition in chemical and biological systems. Angew. Chem. Int. Ed. 2015, 54, 3290–3327. [Google Scholar] [CrossRef] [PubMed]
- Hardegger, L.A.; Kuhn, B.; Spinnler, B.; Anselm, L.; Ecabert, R.; Stihle, M.; Gsell, B.; Thoma, R.; Diez, J.; Benz, J.; et al. Systematic investigation of halogen bonding in protein-ligand interactions. Angew. Chem. Int. Ed. 2010, 50, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, Z.; Liu, Y.; Lu, Y.; Chen, K.; Zhu, W. Halogen bond: Its role beyond drug-target binding affinity for drug discovery and development. J. Chem. Inf. Model. 2014, 54, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. Plip: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.C.; Ho, P.S. Computational tools to model halogen bonds in medicinal chemistry. J. Med. Chem. 2016, 59, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Danelius, E.; Andersson, H.; Jarvoll, P.; Lood, K.; Gräfenstein, J.; Erdélyi, M. Halogen bonding: A powerful tool for modulation of peptide conformation. Biochemistry 2017, 56, 3265–3272. [Google Scholar] [CrossRef] [PubMed]
- Scholfield, M.R.; Ford, M.C.; Carlsson, A.-C.C.; Butta, H.; Mehl, R.A.; Ho, P.S. Structure-energy relationships of halogen bonds in proteins. Biochemistry 2017, 56, 2794–2802. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, A.M.; Pike, A.C.; Dauter, Z.; Hubbard, R.E.; Bonn, T.; Engstöm, O.; Ohman, L.; Greene, G.L.; Gustafsson, J.A.; Carlquist, M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997, 389, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.C.T.; Textor, L.C.; Melo, D.C.; Nascimento, A.S.; Skaf, M.S.; Polikarpov, I. An alternative conformation of ERβ bound to estradiol reveals h12 in a stable antagonist position. Sci. Rep. 2017, 7, 3509. [Google Scholar] [CrossRef] [PubMed]
- Pike, A.; Brzozowski, A.M.; Hubbard, R.E.; Bonn, T.; Thorsell, A.G.; Engström, O.; Ljunggren, J.; Gustafsson, J.K.; Carlquist, M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999, 18, 4608–4618. [Google Scholar] [CrossRef] [PubMed]
- Estébanez-Perpiñá, E.; Moore, J.M.R.; Mar, E.; Delgado-Rodrigues, E.; Nguyen, P.; Baxter, J.D.; Buehrer, B.M.; Webb, P.; Fletterick, R.J.; Guy, R.K. The molecular mechanisms of coactivator utilization in ligand-dependent transactivation by the androgen receptor. J. Biol. Chem. 2005, 280, 8060–8068. [Google Scholar] [CrossRef] [PubMed]
- Arisoy, M. Biodegradation of chlorinated organic compounds by white-rot fungi. Bull. Environ. Contam. Toxicol. 1998, 60, 872–876. [Google Scholar]
- Shah, M.M.; Barr, D.P.; Chung, N.; Aust, S.D. Use of white rot fungi in the degradation of environmental chemicals. Toxicol. Lett. 1992, 64–65, 493–501. [Google Scholar] [CrossRef]
- Takada, S.; Nakamura, M.; Matsueda, T.; Kondo, R.; Sakai, K. Degradation of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans by the white rot fungus phanerochaete sordida yk-624. Appl. Environ. Microbiol. 1996, 62, 4323–4328. [Google Scholar] [PubMed]
- Purnomo, A.S.; Mori, T.; Kamei, I.; Kondo, R. Basic studies and applications on bioremediation of DDT: A review. Int. Biodeterior. Biodegrad. 2011, 65, 921–930. [Google Scholar] [CrossRef]
- Sudharshan, S.; Naidu, R.; Mallavarapu, M.; Bolan, N. Ddt remediation in contaminated soils: A review of recent studies. Biodegradation 2012, 23, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, X.; Luan, S. Uptake and biodegradation of DDT by 4 ectomycorrhizal fungi. Sci. Total Environ. 2007, 385, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Purnomo, A.S.; Ashari, K.; Hermansyah, F.T. Evaluation of the synergistic effect of mixed cultures of white-rot fungus pleurotus ostreatus and biosurfactant-producing bacteria on DDT biodegradation. J. Microbiol. Biotechnol. 2017, 27, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Doddapaneni, H.; Yadav, J.S. Differential regulation and xenobiotic induction of tandem p450 monooxygenase genes PC-1 (CYP63A1) and PC-2 (CYP63A2) in the white-rot fungus phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2004, 65, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Mori, T.; Kamei, I.; Kondo, R. A novel metabolic pathway for biodegradation of ddt by the white rot fungi, Phlebia Lindtneri and Phlebia Brevispora. Biodegradation 2011, 22, 859–867. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Malaria Programme. The Use of DDT in Malaria Vector Control: Who Position Statement. 2011. Available online: http://www.who.int/iris/handle/10665/69945 (accessed on 5 May 2018).
- Glustrom, L.W.; Mitton-Fry, R.M.; Wuttke, D.S. Re: 1,1-dichloro-2,2-bis-(p-chlorophenyl)ethylene and polychlorinated biphenyls and breast cancer: Combined analysis of five U.S. Studies. J. Nat. Cancer Inst. 2002, 94, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Gaido, K.W.; Maness, S.C.; McDonnell, D.P.; Dehal, S.S.; Kupfer, D.; Safe, S. Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: Structure-activity studies. Mol. Pharmacol. 2000, 58, 852–858. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsushima, A. A Novel Action of Endocrine-Disrupting Chemicals on Wildlife; DDT and Its Derivatives Have Remained in the Environment. Int. J. Mol. Sci. 2018, 19, 1377. https://doi.org/10.3390/ijms19051377
Matsushima A. A Novel Action of Endocrine-Disrupting Chemicals on Wildlife; DDT and Its Derivatives Have Remained in the Environment. International Journal of Molecular Sciences. 2018; 19(5):1377. https://doi.org/10.3390/ijms19051377
Chicago/Turabian StyleMatsushima, Ayami. 2018. "A Novel Action of Endocrine-Disrupting Chemicals on Wildlife; DDT and Its Derivatives Have Remained in the Environment" International Journal of Molecular Sciences 19, no. 5: 1377. https://doi.org/10.3390/ijms19051377
APA StyleMatsushima, A. (2018). A Novel Action of Endocrine-Disrupting Chemicals on Wildlife; DDT and Its Derivatives Have Remained in the Environment. International Journal of Molecular Sciences, 19(5), 1377. https://doi.org/10.3390/ijms19051377