Biofilm Inhibition by Novel Natural Product- and Biocide-Containing Coatings Using High-Throughput Screening
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Juglone Leaching
2.2. MIC, EC50, and MBC for Juglone in Solution
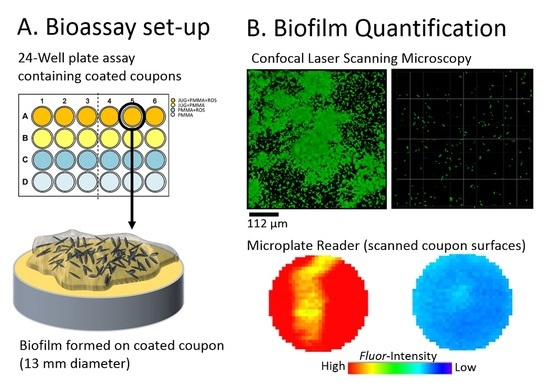
2.3. Assessing Biofilm Growth on Antibiofilm Coatings Using the Microplate Reader
2.4. Planktonic Growth
2.5. Biofilm Morphology
3. Materials and Methods
3.1. Test Surfaces and Coating Formulations
3.2. Leaching of Juglone from Coatings
3.3. Effect of Juglone in Solution against Bacterial Growth (MIC, EC50, and MBC)
3.4. Biofilm Formation on Coatings
3.5. Microscopy and Image Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- IMO, International Shipping and World Trade Facts and Figures. Maritime Knowledge Centre. 2009. Available online: www.vliz.be/imisdocs/publications/234133.pdf (accessed on 13 October 2012).
- Salta, M.; Wharton, J.; Stoodley, P.; Dennington, S.; Goodes, L.; Werwinski, S.; Mart, U.; Wood, R.; Stokes, K. Designing biomimetic antifouling surfaces. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4729–4754. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.G.; Boyd, K.G.; Armstrong, E.; Jiang, Z.; Yan, L.; Berggren, M.; May, U.; Pisacane, A.; Granmo, Å.; Adams, D.R. The development of a marine natural product-based antifouling paint. Biofouling 2003, 19, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.-Y.; Li, Z.; Xu, Y.; Li, Y.; Fusetani, N. Mini-review: Marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 2015, 31, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.H.; Jennings, M.C.; Wuest, W.M. Draining the moat: Disrupting bacterial biofilms with natural products. Tetrahedron 2014, 70, 6373–6383. [Google Scholar] [CrossRef]
- Hellio, C.; De La Broise, D.; Dufossé, L.; LeGal, Y.; Bourgougnon, N. Inhibition of marine bacteria by extracts of macroalgae: Potential use for environmentally friendly antifouling paints. Mar. Environ. Res. 2001, 52, 231–247. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R.; Briand, J.-F. Marine biofilms on artificial surfaces: Structure and dynamics. Environ. Microbiol. 2013, 15, 2879–2893. [Google Scholar] [CrossRef] [PubMed]
- Briand, J.F. Marine antifouling laboratory bioassays: An overview of their diversity. Biofouling 2009, 25, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Salta, M.; Chambers, L.; Wharton, J.; Wood, R.; Briand, J.-F.; Blache, Y.; Stokes, K. Marine fouling organisms and their use in antifouling bioassays. In Proceedings of the EUROCORR, Nice, France, 6–9 September 2009. [Google Scholar]
- Stafslien, S.; Bahr, J.; Feser, J.; Weisz, J.; Chisholm, B.; Ready, T.; Boudjouk, P. Combinatorial materials research applied to the development of new surface coatings I: A multiwell plate screening method for the high-throughput assessment of bacterial biofilm retention on surfaces. J. Comb. Chem. 2006, 8, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Stafslien, S.; Daniels, J.; Chisholm, B.; Christianson, D. Combinatorial materials research applied to the development of new surface coatings III. Utilisation of a high-throughput multiwell plate screening method to rapidly assess bacterial biofilm retention on antifouling surfaces. Biofouling 2007, 23, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Leroy, C.; Delbarre Ladrat, C.; Ghillebaert, F.; Rochet, M.; Compere, C.; Combes, D. A marine bacterial adhesion microplate test using the DAPI fluorescent dye: A new method to screen antifouling agents. Lett. Appl. Microbiol. 2007, 44, 372–378. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, F.; Bruin, A.; Biersteker, R.; Donnelly, G.; Klijnstra, J.; Rentrop, C.; Willemsen, P. Bacterial assay for the rapid assessment of antifouling and fouling release properties of coatings and materials. J. Ind. Microbiol. Biotechnol. 2010, 37, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Camps, M.; Briand, J.-F.O.; Guentas-Dombrowsky, L.; Culioli, G.R.; Bazire, A.; Blache, Y. Antifouling activity of commercial biocides vs. natural and natural-derived products assessed by marine bacteria adhesion bioassay. Mar. Pollut. Bull. 2011, 62, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Salta, M.; Wharton, J.A.; Dennington, S.P.; Stoodley, P.; Stokes, K.R. Anti-Biofilm Performance of Three Natural Products against Initial Bacterial Attachment. Int. J. Mol. Sci. 2013, 14, 21757–21780. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, H.; Ikigai, H.; Yoshitake, M. Evaluation of various metallic coatings on steel to mitigate biofilm formation. Int. J. Mol. Sci. 2009, 10, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, A.; Spangehl, M. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin. Orthop. Relat. Res. 2004, 427, 79–85. [Google Scholar] [CrossRef]
- Zalavras, C.; Patzakis, M.; Holtom, P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin. Orthop. Relat. Res. 2004, 427, 86–93. [Google Scholar] [CrossRef]
- Mori, R.; Nakai, T.; Enomoto, K.; Uchio, Y.; Yoshino, K. Increased Antibiotic Release from a Bone Cement Containing Bacterial Cellulose. Clin. Orthop. Relat. Res. 2010, 469, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Kuechle, D.; Landon, G.; Musher, D.; Noble, P. Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin. Orthop. Relat. Res. 1991, 264, 302–308. [Google Scholar] [CrossRef]
- Méndez, J.; Abraham, G.; Fernández, M.; Vázquez, B.; Román, J. Self curing acrylic formulations containing PMMA/PCL composites: Properties and antibiotic release behavior. J. Biomed. Mater. Res. 2002, 61, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Virto, M.; Frutos, P.; Torrado, S.; Frutos, G. Gentamicin release from modified acrylic bone cements with lactose and hydroxypropylmethylcellulose. Biomaterials 2003, 24, 79–87. [Google Scholar] [CrossRef]
- Lewis, G. Properties of antibiotic loaded acrylic bone cements for use in cemented arthroplasties: A state of the art review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 558–574. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, P.; Bhosle, N. Bacterial extracellular polymeric substance (EPS): A carrier of heavy metals in the marine food-chain. Environ. Int. 2006, 32, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Howell, D. Testing the impact of biofilms on the performance of marine antifouling coatings. In Advances in Marine Antifouling Coatings and Technologies; Hellio, C., Yebra, D., Eds.; Woodhead: Cambridge, UK, 2009. [Google Scholar]
- Ekblad, T.; Bergstrom, G.; Ederth, T.; Conlan, S.; Mutton, R.; Clare, A.; Wang, S.; Liu, Y.; Zhao, Q.; D’Souza, F.; et al. Poly(ethylene glycol)-containing hydrogel surfaces for antifouling applications in marine and freshwater environments. Biomacromolecules 2008, 9, 2775–2783. [Google Scholar] [CrossRef] [PubMed]
- Akesso, L.; Pettitt, M.; Callow, J.; Callow, M.; Stallard, J.; Teer, D.; Liu, C.; Wang, S.; Zhao, Q.; Willemsen, P. The potential of nano-structured silicon oxide type coatings deposited by PACVD for control of aquatic biofouling. Biofouling 2009, 25, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersboll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Chobot, V.; Hadacek, F. Milieu-dependent pro-and antioxidant activity of juglone may explain linear and nonlinear effects on seedling development. J. Chem. Ecol. 2009, 35, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.M.; El-Saeed, S.M.; Farag, R.K. New vinyl ester resins based on rosin for coating applications. React. Funct. Polym. 2006, 66, 1596–1608. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5. [Google Scholar] [CrossRef]
- Kugel, A.; Stafslien, S.; Chisholm, B.J. Antimicrobial coatings produced by “tethering” biocides to the coating matrix: A comprehensive review. Progress Org. Coat. 2011, 72, 222–252. [Google Scholar] [CrossRef]
- Scardino, A.J.; de Nys, R. Mini review: Biomimetic models and bioinspired surfaces for fouling control. Biofouling 2011, 27, 73–86. [Google Scholar] [CrossRef] [PubMed]








| Coating | 24 h | 48 h | Coating Thickness (μm) | p-Value |
|---|---|---|---|---|
| Juglone conc. (ppm) | ||||
| Coating 1 | 1.70 ± 0.20 | 1.96 ± 0.21 | 14.95 ± 2.02 | p < 0.05 |
| Coating 2 | 1.99 ± 0.36 | 2.35 ± 0.35 | 19.57 ± 6.05 | NS |
| p-value | NS | p < 0.04 | ||
| Formulations | Coating No. | Coating Formulation | Biofilm Max. Thickness (μm) | Biofilm Biovolume (μm3 μm–2) |
|---|---|---|---|---|
| NP coating | 1 | JUG + PMMA + ROS | 12.30 ± 0.60 | 0.09 ± 0.04 |
| 2 | JUG + PMMA | 12.50 ± 2.30 | 0.04 ± 0.02 | |
| Controls | 3 | PMMA + ROS | 33.50 ± 5.20 | 8.60 ± 2.17 |
| 4 | PMMA | 23.00 ± 2.00 | 1.10 ± 0.49 | |
| Biocide coating | 5 | Cu2O + PMMA + ROS | 10.90 ± 0.60 | 0.01 ± 0.00 |
| 6 | Cu2O + PMMA | 09.80 ± 0.70 | 0.01 ± 0.00 | |
| PKW value | 1 vs. 3 * vs. 5 | p < 0.001 | p < 0.001 | |
| PKW value | 2 vs. 4 * vs. 6 | p < 0.001 | p < 0.002 | |
| PKW value | 1 vs. 2 | NS | NS | |
| PKW value | 3 vs. 4 | p < 0.043 | p < 0.005 | |
| PKW value | 5 vs. 6 | NS | NS |
| Formulation | Coating No. | Coating Formulation | Coating Composition | Active Compound Concentration (μg/g) | Coating Thickness (μm) | Total No. of Samples | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (wt. %) | (vol. %) | ||||||||||
| AC * | PMMA | ROS | AC * | PMMA | ROS | ||||||
| NP coatings | 1 | JUG + PMMA +ROS | 6.25 | 75 | 18.75 | 5 | 76 | 19 | 62,500 | 15.03 ± 4.2 | 12 |
| 2 | JUG + PMMA | 6.15 | 93.85 | 5 | 95 | 61,500 | 14.78 ± 4.2 | 12 | |||
| Biocide coatings | 3 | Cu2O + PMMA + ROS | 68.91 | 24.87 | 6.22 | 30 | 56 | 14 | 689,100 | 7.87 ± 3.0 | 12 |
| 4 | Cu2O + PMMA | 68.55 | 34.45 | 30 | 70 | 685,500 | 4.45 ± 0.7 | 12 | |||
| Controls | 5 | PMMA + ROS | 0 | 80 | 20 | 0 | 80 | 20 | 0 | 3.91 ± 0.7 | 24 |
| 6 | PMMA | 0 | 100 | 0 | 100 | 0 | 3.81 ± 0.6 | 24 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salta, M.; Dennington, S.P.; Wharton, J.A. Biofilm Inhibition by Novel Natural Product- and Biocide-Containing Coatings Using High-Throughput Screening. Int. J. Mol. Sci. 2018, 19, 1434. https://doi.org/10.3390/ijms19051434
Salta M, Dennington SP, Wharton JA. Biofilm Inhibition by Novel Natural Product- and Biocide-Containing Coatings Using High-Throughput Screening. International Journal of Molecular Sciences. 2018; 19(5):1434. https://doi.org/10.3390/ijms19051434
Chicago/Turabian StyleSalta, Maria, Simon P. Dennington, and Julian A. Wharton. 2018. "Biofilm Inhibition by Novel Natural Product- and Biocide-Containing Coatings Using High-Throughput Screening" International Journal of Molecular Sciences 19, no. 5: 1434. https://doi.org/10.3390/ijms19051434
APA StyleSalta, M., Dennington, S. P., & Wharton, J. A. (2018). Biofilm Inhibition by Novel Natural Product- and Biocide-Containing Coatings Using High-Throughput Screening. International Journal of Molecular Sciences, 19(5), 1434. https://doi.org/10.3390/ijms19051434