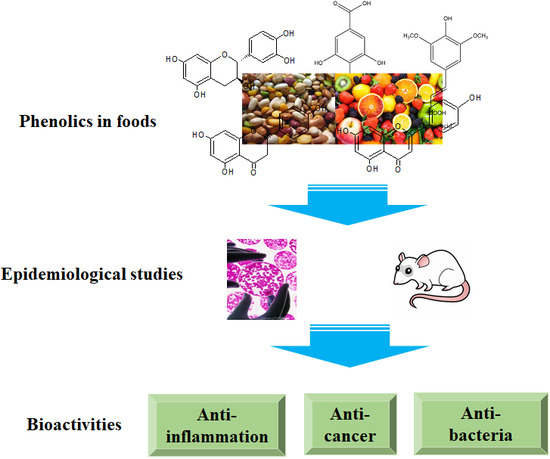
Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review
Abstract
:1. Introduction
1.1. Phenolics
1.2. Classification of Phenolics
1.3. Biosynthesis of Phenolics
1.4. Transfer of Phenolics
1.5. Absorption of Phenolics in the Digestive Tract
2. Bioactivities of Standard Phenolics
2.1. Anticancer
2.2. Anti-Inflammatory Activity
2.3. Antibacterial and Anti-Viral Activity
2.4. Other Bioactivities of Phenolics
3. Bioactivities of Phenolic Extracts from Natural Origin
3.1. Grains/Cereals
3.2. Legumes/Seeds
3.3. Fruits/Vegetables
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Cirillo, E.; Natella, F.; Scaccini, C. Absorption of phenolic acids in humans after coffee consumption. J. Agric. Food Chem. 2002, 50, 5735–5741. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, J.; Mishima, M.; Kurihara, H.; Mizuntani, J.; Kobophenol, B. A tetrastilbene from Carex pumila. Phytochemistry 1991, 2, 645–647. [Google Scholar] [CrossRef]
- Yamada, M.; Hayashi, K.I.; Hayashi, H.; Ikeda, S.; Hoshino, T.; Tsutsui, K.; Tsutsui, K.; Iinuma, M.; Nozaki, H. Stilbenoids from Kobresia nepalensis exhibiting DNA topoisomerase II inhibition. Phytochemistry 2006, 67, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.; Shafique, A.; Dore, S. Protective effects of resveratrol in age-related neurodegenerative diseases and gene regulatory action. In Oxidative Stress and Diseases: Resveratrol in Health and Disease; Aggarwal, B.B., Shishodia, S., Packer, L., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 499–518. [Google Scholar]
- Han, Y.; Bastianoetto, S.; Quirion, R. Neuroprotective effects of resveratrol. In Oxidative Stress and Diseases: Resveratrol in Health and Disease; Aggarwal, B.B., Shishodia, S., Packer, L., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 619–630. [Google Scholar]
- Lacy, A.; O’Kennedy, R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Joshi, H. Coumarin: Chemical and pharmacological profile. J. Appl. Pharm. Sci. 2012, 2, 236–240. [Google Scholar]
- Lee, S.J.; Lee, U.S.; Kim, W.J.; Moon, S.K. Inhibitory effect of esculetin on migration, invasion and matrix metalloproteinase-9 expression in TNF-α-induced vascular smooth muscle cells. Mol. Med. Rep. 2011, 4, 337–341. [Google Scholar] [PubMed]
- Nitiema, L.W.; Savadogo, A.; Simpore, J.; Dianou, D.; Traore, A.S. In vitro antimicrobial activity of some phenolic compounds (coumarin and quercetin) against gastroenteritis bacterial strains. Int. J. Microbiol. Res. 2012, 3, 183–187. [Google Scholar]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Pramar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Singh, B.; Singh, N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorgan. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Alshikh, N.; de Camargo, A.C.; Shahidi, F. Phenolics of selected lentil cultivars: Antioxidant activities and inhibition of low-density lipoprotein and DNA damage. J. Funct. Foods 2015, 18, 1022–1038. [Google Scholar] [CrossRef]
- De Freitas, V.; Glories, Y. Concentration and compositional changes of procyanidins in grape seeds and skin of white Vitis Vinifera varieties. J. Sci. Food Agric. 1999, 79, 1601–1606. [Google Scholar] [CrossRef]
- Pérez-Ilzarbe, F.J.; Martinez, V.; Hernández, T.; Estrella, I. Liquid chromatographic determination of apple pulp procyanidins. J. Liq. Chromatogr. 1992, 15, 637–646. [Google Scholar] [CrossRef]
- Saito, M.; Hosoyama, H.; Ariga, T.; Kataoda, S.; Yamaji, N. Antiulcer activity of grape seed extract and procyanidins. J. Agric. Food Chem. 1998, 46, 1460–1464. [Google Scholar] [CrossRef]
- Castillo, J.; Benavente-Garcia, O.; Lorente, J.; Alcaraz, M.; Redondo, A.; Ortunõ, A.; del Rio, J.A. Antioxidant activity and radioprotective effects against chromosomal damage induced in vivo by X-rays of flavan-3-ols (procyanidins) froma grape seeds (Vitis Vinifera): Comparative study versus other phenolic and organic compounds. J. Agric. Food Chem. 2000, 48, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Ricardo da Silva, J.M.; Darmon, N.; Fernández, U.; Mitjavila, S. Oxygen free radical scavenger capacity in aqueous models of different procyanidins from grape seeds. J. Agric. Food Chem. 1991, 39, 1549–1552. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J.D. Review: Insoluble-bound phenolics in foods. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press Inc.: Boca Raton, FL, USA, 2004; pp. 13–141. [Google Scholar]
- Podsedek, A.; Redzynia, M.; Klewicka, E.; Koziołkiewicz, M. Matrix effects on the stability and antioxidant activity of red cabbage anthocyanins under simulated gastrointestinal digestion. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073–2085. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.T. Intestinal release and uptake of phenolic antioxidant diferulic acids. Free Radic. Biol. Med. 2001, 31, 304–314. [Google Scholar] [CrossRef]
- Gibson, G.; Roberfroid, M. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Yi, W.; Fisher, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.D.; Chen, J.C.; Lai, T.Y.; Yang, J.S.; Yu, C.S.; Chiang, J.H.; Lu, C.C.; Yang, S.T.; Yu, C.C.; Chang, S.J.; et al. Gallic acid induces G(0)/G(1) phase arrest and apoptosis in humanleukemia HL-60 cells through inhibiting cyclin D and E, and activating mitochondria-dependent pathway. Anticancer Res. 2011, 31, 2821–2832. [Google Scholar] [PubMed]
- Veluri, R.; Singh, R.P.; Liu, Z.; Thompson, J.A.; Agarwal, R.; Agarwal, C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and poptotic death of DU-145 human prostate carcinoma cells. Carcinogenes 2006, 27, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Kamatham, S.; Kumar, N.; Gudipalli, P. Isolation and characterization of gallic acid and methylgallate from the seed coats of Givotia rottleriformis Griff. and their anti-proliferative effect on human epidermoidcarcinoma A431 cells. Toxicol. Rep. 2015, 2, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Fahrioğlu, U.; Dodurga, Y.; Elmas, L.; Seçme, M. Ferulic acid decreases cell viability and colony formation while inhibiting migration of MIA PaCa-2 human pancreatic cancer cells in vitro. Gene 2016, 576, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Eitsuka, T.; Tatewaki, N.; Nishida, H.; Kurata, T.; Nakagawa, K.; Miyazawa, T. Synergistic inhibition of cancer cell proliferation with a combination of delta-tocotrienol and ferulic acid. Biochem. Biophys. Res. Commun. 2014, 453, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.E.; Park, E. Ferulic acid in combination with PARP inhibitor sensitizes breast cancer cells as chemotherapeutic strategy. Biochem. Biophys. Res. Commun. 2015, 458, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Janicke, B.; Hegardt, C.; Krogh, M.; Onning, G.; Akesson, B.; Cirenajwis, H.M.; Oredsson, S.M. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutr. Cancer 2011, 63, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.Y.; Wang, H.M.; Chang, K.F.; Hu, H.T.; Hwang, L.J.; Fu, T.F.; Chen, B.H. Feruloyl-L-arabinose attenuates migration, invasion and production of reactive oxygen species in H1299 lung cancer cells. Food Chem. Toxicol. 2013, 58, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Panat, N.A.; Singh, B.G.; Maurya, D.K.; Sandur, S.K.; Ghaskadbi, S.S. Troxerutin, a natural flavonoid binds to DNA minor groove and enhances cancer cell killing in response to radiation. Chem. Biol. Interact. 2016, 251, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Hirayama, R.; Kubota, N. The chemopreventive flavonoid apigenin confers radiosensitizing effect in human tumor cells grown as monolayers and spheroids. J. Radiat. Res. 2007, 48, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.W.C.; Lin, C.J.; Hour, M.J.; Yang, W.H.; Wang, M.Y.; Lee, H.Z. Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem. Toxicol. 2007, 45, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, P.; Ramanathan, M. Effect of myricetin on 1,2 dimethylhydrazineinduced rat colon carcinogenesis. J. Exp. Ther. Oncol. 2011, 9, 101–108. [Google Scholar] [PubMed]
- Hsu, W.C.; Chang, S.P.; Lin, L.C.; Li, C.L.; Richardson, C.D.; Lin, C.C.; Lin, L.T. Limonium sinense and gallic acid suppress hepatitis C virus infection by blocking early viral entry. Antivir. Res. 2015, 118, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Oh, J.S.; Kang, I.C.; Hong, S.J.; Choi, C.H. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008, 46, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Kang, O.H.; Lee, Y.S.; Oh, Y.C.; Chae, H.S.; Jang, H.J.; Kim, J.H.; Sohn, D.H.; Shin, D.W.; Park, H.; et al. In vitro activity of methyl gallate isolated from galla rhois alone and in combination with ciprofloxacin against clinical isolates of salmonella. J. Microbiol. Biotechnol. 2008, 18, 1848–1852. [Google Scholar] [PubMed]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid regulates hepatic GLUT2 gene expression in high fat and fructose-induced type-2 diabetic adult male rat. Eur. J. Pharmacol. 2015, 761, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.Z.; Zhao, G.R.; Yang, J.; Yuan, Y.J.; Zhu, G.G.; Hiltunen, R. Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sci. 2004, 75, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, O.; Mandel, S.; Amit, T.; Moussa, B.; Youdim, H. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J. Nutr. Biochem. 2004, 15, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Borenstein, A.R.; Wu, Y. Fruit and vegetable juices and Alzheimer’s disease: The Kame project. Am. J. Clin. Nutr. 2006, 119, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.; Kuo, Y.C.; Chou, C.J. Anti-herpes simplex virus type-1 flavonoids and a new flavanone from the root of Limonium sinense. Planta Med. 2000, 66, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Kumar, S.; Kumar, A.; Siddiqui, J.A.; Swarnkar, G.; Gupta, V.; Kendurker, A.; Dwivedi, A.K.; Romero, J.R.; Chattopadhyay, N. Kaempferol has osteogenic effect in ovariectomized adult Spraguee Dawley rats. Mol. Cell. Endocrinol. 2008, 289, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; Graziani, I.; De Zio, D.; Dini, L.; Centonze, D.; Rotilio, G.; Ciriolo, M.R. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: Possible implications for Parkinson’s disease. Neurobiol. Aging 2012, 33, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A review on the dietary flavonoid kaempferol. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.M.; Kang, J.G.; Bae, J.S.; Pae, H.O.; Lyu, Y.S.; Jeon, B.H. The flavonoid apigenin ameliorates cisplatin-induced nephrotoxicity through reduction of p53 activation and promotion of PI3K/Akt pathway in human renal proximal tubular epithelial cells. Evid. Based Complement. Altern. Med. 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, Y.; Aouey, B.; Aroui, S.; Kebieche, M.; Fetoui, H. Anti-apoptotic and anti-inflammatory effects of naringin on cisplatin-induced renal injury in the rat. Chem. Biol. Interact. 2016, 243, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kamel, K.M.; El-Raouf, A.; Ola, M.; Metwally, S.A.; El-Latif, A.; Hekma, A.; Elsayed, M.E. Hesperidin and rutin, antioxidant citrus flavonoids, attenuate cisplatin-induced nephrotoxicity in rats. J. Biochem. Mol. Toxic. 2014, 28, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.S.; Huang, G.J.; Lu, Y.H.; Chang, L.W. Anti-inflammatory effects of an aqueous extract of Welsh onion green leaves in mice. Food Chem. 2013, 138, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Mong, M.C.; Chan, K.C.; Yin, M.C. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, F.M.; Duma, D.; Assreuy, J.; Buzzi, F.C.; Niero, R.; Campos, M.M.; Calixto, J.B. Caffeic acid derivatives: In vitro and in vivo anti-inflammatory properties. Free Radic. Res. 2004, 38, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Hamalainen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007. [Google Scholar] [CrossRef]
- Kane, C.J.; Menna, J.H.; Sung, C.C.; Yeh, Y.C. Methyl gallate, methyl-3,4,5-trihydoxybenzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. II. Antiviral activity of methyl gallate and its derivatives. Biosci. Rep. 1988, 8, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Alvesalo, J.; Vuorela, H.; Tammela, P.; Leinonen, M.; Saikku, P.; Vuorela, P. Inhibitory effect of dietary phenolic compounds on Chlamydia pneumoniae in cell cultures. Biochem. Pharmacol. 2006, 71, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, N.; Iwamaru, Y.; Yahiro, K.; Tagashira, M.; Moss, J.; Noda, M. Differential activities of plant polyphenols on the binding and internalization of cholera toxin in vero cells. J. Biol. Chem. 2005, 280, 23303–23309. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; De Villiers, W.J.; McClain, C.J.; Varilek, G.W. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J. Nutr. 1998, 128, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, E.; Menegazzi, M.; Yao, Y.; Suzuki, H.; Forstermann, U.; Kleinert, H. Green tea inhibits human inducible nitric-oxide synthase expression by down-regulating signal transducer and activator of transcription-1α activation. Mol. Pharmacol. 2004, 65, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Kuzuya, M.; Cheng, X.W.; Asai, T.; Kanda, S.; Tamaya-Mori, N.; Sasaki, T.; Shibata, T.; Iguchi, A. Green tea catechins inhibit the cultured smooth muscle cell invasion through the basement barrier. Atherosclerosis 2003, 166, 23–30. [Google Scholar] [CrossRef]
- Larson, A.J.; Symons, J.D.; Jalili, T. Therapeutic potential of quercetin to decrease blood pressure: Review of efficacy and mechanisms. Adv. Nutr. 2012, 3, 39–46. [Google Scholar] [CrossRef] [PubMed]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Pirola, L.; Frojdo, S. Resveratrol: One molecule, many targets. IUBMB Life 2008, 60, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Ueda, T.; Oki, T.; Sugita, K.; Terahara, N.; Matsumoto, K. α-Glucosidase inhibitory action of natural acylated anthocyanins. J. Agric. Food Chem. 2001, 49, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Holbrook, J.T.; Wise, R.; Blumenthal, M.; Dozor, A.J.; Mastronarde, J. Dietary intake of soy genistein is associated with lung function in patients with asthma. J. Asthma 2004, 41, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, D.; Kim, C.S.; Oh, T.W.; Yang, C.Y.; Naka, T.; Igawa, S.; Ohta, F. Suppressive effects of genistein dosage and resistance exercise on bone loss in ovariectomized rats. J. Physiol. Anthropol. Appl. Hum. Sci. 2001, 20, 285–291. [Google Scholar] [CrossRef]
- Sharma, V.; Rao, L.J. A thought on the biological activities of black tea. Crit. Rev. Food Sci. Nutr. 2009, 49, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Uchida, K.; Hiromitsu, A.; Osawa, T. Dietary cyanidin 3-O-β-d-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Cichewicz, R.H.; Chandra, A.; Nair, M.G. Cyclooxygenase inhibitory and antioxidant compounds from crabapple fruits. J. Agric. Food Chem. 2003, 51, 1948–1951. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T. Tannins, a new family of bio-active natural organic compounds questions and answers. J. Pharm. Soc. Jpn. 1995, 115, 81–100. [Google Scholar] [CrossRef]
- Liu, S.; Manson, J.E.; Stamfer, M.J.; Hu, F.B.; Giovannucci, E.; Colditz, G.A. A prospective study of whole-grain intake and risk of type 2 diabetes mellitus in US women. Am. J. Public Health 2000, 90, 1409–1415. [Google Scholar] [PubMed]
- Lloyd, B.J.; Siebenmorgen, T.J.; Beers, K.W. Effects of commercial processing on antioxidants in rice bran. Cereal Chem. 2000, 77, 551–555. [Google Scholar] [CrossRef]
- Topping, D. Cereal complex carbohydrates and their contribution to human health. J. Cereal Sci. 2007, 46, 220–229. [Google Scholar] [CrossRef]
- Chen, Q.; Ling, W.H.; Ma, J.; Mei, J. Effects of black and red rice on the formation of aortic plaques and blood lipids in rabbits. J. Hyg. Res. 2000, 29, 170–172. [Google Scholar]
- Koide, T.; Kamei, H.; Hashimoto, Y.; Kojima, T.; Hasegawa, M. Antitumor effect of hydrolyzed anthocyanin from grape rinds and red rice. Cancer Biother. Radiopharm. 1996, 11, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kang, C.S.; Lee, E.H.; Shin, T.Y. The evaluation of the antianaphylactic effect of Oryza sativa L. subsp. hsien Ting in rats. Pharmacol. Res. 1999, 40, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.L. A research report on the pharmacologic test of black rice pigment. J. Wuhan Food Ind. Coll. 1997, 3, 10–12. [Google Scholar]
- Chandrasekara, A.; Shahidi, F. Bioactivities and antiradical properties of millet grains and hulls. J. Agric. Food Chem. 2011, 59, 9563–9571. [Google Scholar] [CrossRef] [PubMed]
- Surendrian, G.; Goh, C.; Le, K.; Zhao, Z.; Askarian, F.; Othman, R.; Moghadasian, M.H. Wild rice (Zizania paplustris L.) prevents atherogenesis in LDL receptor knockout mice. Atherosclerosis 2013, 230, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, R.H.; Lien, L.M.; Lin, S.H.; Chen, C.W.; Cheng, H.J.; Cheng, H.H. Alleviation of oxidative damage in multiple tissues in rats with streptozotocin induced diabetes by rice bran oil supplementation. Ann. N. Y. Acad. Sci. 2005, 1042, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Sergent, T.; Vanderstraeten, J.; Winand, J.; Beguin, P.; Schneider, Y. Phenolic compounds and plant extracts as potential natural antiobesity substances. Food Chem. 2012, 135, 68–73. [Google Scholar] [CrossRef]
- Stanisavljević, N.S.; Ilić, M.D.; Matić, I.Z.; Jovanović, Ž.S.; Čupić, T.; Dabić, D.Č.; Natić, M.M.; Tešić, Ž.L. Identification of phenolic compounds from seed coats of differently colored european varieties of pea (Pisum sativum L.) and characterization of their antioxidant and In vitro anticancer activities. Nutr. Cancer 2016, 68, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.U.; Rickard, S.E.; Cheung, F.; Kenaschuk, E.O.; Obermeyer, W.R. Variability in anticancer lignan levels in flaxseed. Nutr. Cancer 1997, 27, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Sohn, D.W.; Bae, W.J.; Kim, H.S.; Kim, S.W.; Kim, S.W. The anti-inflammatory and antifibrosis effects of anthocyanin extracted from black soybean on a Peyronie disease rat model. Urology 2014, 84, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Monk, J.M.; Lu, J.T.; Zarepoor, L.; Wu, W.; Liu, R.; Pauls, K.P.; Wood, G.A.; Robinson, L.; Tsao, R.; et al. Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br. J. Nutr. 2014, 111, 1549–1563. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, K.C.; Fang, M.; Lim, Y.C.; Jeon, Y.M.; Lee, J.C. Red bean extract reduces inflmmation and increases survival murine sepsis model. Food Sci. Biotechnol. 2011, 20, 1125–1131. [Google Scholar] [CrossRef]
- Patterson, L.H.; Murray, G.I. Tumour cytochrome P450 and drugactivation. Curr. Pharm. Des. 2002, 8, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2017, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Spertini, L.; Parpinel, M.; Gnagnarella, P.; Lagiou, P.; Negri, E.; Franceschi, S.; Montella, M.; Peterson, J.; Dwyer, J.; et al. Flavonoids and breast cancer risk in Italy. Cancer Epidemiol. Biomark. Prev. 2005, 14, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Lagiou, P.; Samoli, E.; Lagiou, A.; Katsouyanni, K.; La Vecchia, C.; Dwyer, J.; Trichopoulos, D. Flavonoid intake and breast cancer risk: A case–control study in Greece. Br. J. Cancer 2003, 89, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Matias, A.A.; Frade, R.F.M.; Duarte, R.O.; Feliciano, R.P.; Bronze, M.R. Characterization of traditional and exotic apple varieties from Portugal. Part 2-Antioxidant and anti-proliferative activities. J. Funct. Foods 2010, 2, 46–53. [Google Scholar] [CrossRef]
- Ha, S.K.; Park, H.Y.; Eom, H.; Kim, Y.; Choi, I. Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-κB and MAPKs activation. Food Chem. Toxicol. 2012, 50, 3498–3504. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, E.P. A berry thought-provoking idea: The potential role of plant polyphenols in the treatment of age-related cognitive disorders. Br. J. Nutr. 2012, 108, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Giampieri, F.; Forbes, T.; Battino, M. Strawberry extracts efficiently counteract inflammatory stress induced by the endotoxin lipopolysaccharide in Human Dermal Fibroblast. Food Chem. Toxicol. 2018, 114, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Visioli, F. Polyphenols and health: Moving beyond antioxidants. J. Berry Res. 2012, 2, 63–67. [Google Scholar]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, U.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]



| Bioactivity | Reference | |
|---|---|---|
| Phenolic Acid | ||
| Gallic acid | Anticancer HCV inhibition Antibacterial | [30,31] [42] [43,44] |
| Methyl gallate | Anticancer | [32] |
| p-Coumaric acid | Anticancer | [36] |
| Ferulic acid | Anticancer Alleviates angina pectoris Reducing hypertension Reducing type 2-diabetes | [33,45] [46] [46] [45] |
| Chlorogenic and Caffeic acids | Anti-mutagenic and-carcinogenic activity | [47] |
| Flavonoid | ||
| Catechin | Preventing Parkinson’s and Alzheimer’s diseases | [48,49] |
| (+)-Epigallocatechin 3-O-gallate | Anti-virus | [50] |
| Quercetin | Anticancer | [51] |
| Kaempferol | Anticancer Anti-inflammation Osteoporotic activity | [40,52,53] [54] [53] |
| Myricetin | Anticancer | [41] |
| Apigenin | Anticancer Anti-inflammation | [39] [55] |
| Troxerutin | Anticancer | [38] |
| Naringin | Anti-inflammation | [56] |
| Hesperidin | Anti-inflammation | [57] |
| Rutin | Anti-inflammation | [57] |
| Phenolics | Bioactivities | References | |
|---|---|---|---|
| Grains/Cereals | |||
| Black rice | Flavones, tannin, and anthocyanidins | Anti-atherosclerosis activity Antitumor activity Anti-allergic activity Anti-fatigue and hypoxia tolerance | [86] [87] [88] [89] |
| Millet | Phenolic acids, flavonoids, and proanthocyanidins | Inhibition of radical-induced DNA scission and the oxidation of human LDL cholesterol | [90] |
| Wild rice | Phenolics | Enhancing SOD and CAT activities | [91] |
| Rye | Ferulic acid | Decrease in mtDNA 8-OhdG levels in liver, kidneys, and pancreas | [92] |
| Legumes/Seeds | |||
| Grape seed | Kaempferol and quercetin | Inhibition of lipase activity | [93] |
| Pea (seed coats) | Phenolic acids and flavonoids | Anticancer | [94] |
| Flaxseed | Lignan | Anticancer | [95] |
| Lentil | Phenolic acids, flavonoids, and proanthocyanidins | Inhibition of radical-induced DNA scission and the oxidation of human LDL cholesterol | [15] |
| Black soybean | Anthocyanins | Anti-inflammation | [96] |
| Navy and black bean | Phenolic acids, flavonoids, and anthocyanins | Anti-inflammation | [97] |
| Red bean | Catechin-7-β-d-glucopyranoside | Anti-inflammation | [98] |
| Fruits | |||
| Pomegranate juice | Phenolic acids, flavonoids, and proanthocyanidins | Anticancer Anti-inflammation | [99] [100] |
| Blackberry | Phenolic acids, flavonoids, and tannins | Anticancer | [101] |
| Indian gooseberry | Phenolic acids and flavonoids | Anticancer | [101] |
| Orange | Flavonoids | Anticancer (breast cancer) | [102] [103] |
| Apple | Catechin, procyanidins, and phloridzin | Anticancer | [104] |
| Citrus | Narirutin | Anti-inflammation | [105] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. https://doi.org/10.3390/ijms19061573
Shahidi F, Yeo J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. International Journal of Molecular Sciences. 2018; 19(6):1573. https://doi.org/10.3390/ijms19061573
Chicago/Turabian StyleShahidi, Fereidoon, and JuDong Yeo. 2018. "Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review" International Journal of Molecular Sciences 19, no. 6: 1573. https://doi.org/10.3390/ijms19061573
APA StyleShahidi, F., & Yeo, J. (2018). Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. International Journal of Molecular Sciences, 19(6), 1573. https://doi.org/10.3390/ijms19061573