Tumor Size-Dependent Anticancer Efficacy of Chlorin Derivatives for Photodynamic Therapy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Prepared Photosensitizers
2.2. In Vitro Phototoxicity
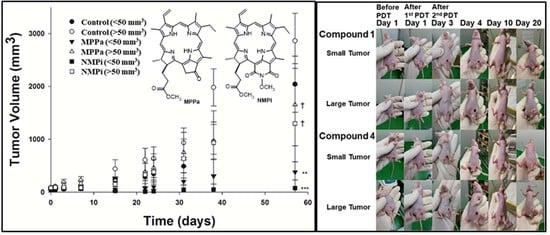
2.3. In Vivo Photodynamic Therapeutic Efficacy
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Photosensitizers
3.3. In Vitro Phototoxicity
3.3.1. MTT Assay According to Concentration of Photosensitizer
3.3.2. CCK-8 Assay According to Irradiation Time and Concentration of Photosensitizer
3.4. Microscopic Analysis
3.5. Singlet Oxygen Photogeneration Study
3.6. FACS Analysis
3.7. Animals and Tumor Model
3.8. In Vivo Anticancer Efficacy in Tumor-Bearing Mice
3.9. Histology Examination
3.10. TUNEL Assay for Apoptosis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- The Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar]
- Sharouni, S.Y.E.; Kal, H.B.; Battermann, J.J. Accelerated regrowth of non-small-cell lung tumours after induction chemotherapy. Br. J. Cancer 2003, 89, 2184–2189. [Google Scholar] [CrossRef] [PubMed]
- Kostron, H.; Hasan, T. (Eds.) Photodynamic Medicine: From Bench to Clinic; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Pandey, R.K.; Kessel, D.; Dougherty, T.J. (Eds.) Handbook of Photodynamic Therapy: Updates on Recent Applications of Porphyrin-Based Compounds; World Scientific Publishing: Singapore, 2016. [Google Scholar]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Marcus, S.L. Photodynamic therapy. Eur. J. Cancer 1992, 28a, 1734–1742. [Google Scholar] [CrossRef]
- Pass, H.I. Photodynamic therapy in oncology: Mechanisms and clinical use. J. Natl. Cancer Inst. 1993, 85, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Folkes, L.K.; Wardman, P. Enhancing the efficacy of photodynamic cancer therapy by radicals from plant auxin (indole-3-acetic acid). Cancer Res. 2003, 63, 776–779. [Google Scholar] [PubMed]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Moghissi, K.; Dixon, K.; Thorpe, J.A.C.; Stringer, M.; Oxtoby, C. Photodynamic therapy (PDT) in early central lung cancer: A treatment option for patients ineligible for surgical resection. Thorax 2007, 62, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-Y.; Wu, F.-Y.; Yang, M.-K.; Guo, Y.-M.; Lu, G.-H.; Yang, Y.-H. A classic near-infrared probe indocyanine green for detecting singlet oxygen. Int. J. Mol. Sci. 2016, 17, 219. [Google Scholar] [CrossRef] [PubMed]
- Bazylińska, U.; Frąckowiak, R.; Brzózka, Z.; Wilk, K.A. The effect of anionic dicephalic surfactants on fabrication of varied-core nanocarriers for sustained release of porphyrin photosensitizers. J. Photochem. Photobiol. B Biol. 2017, 166, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Bellnier, D.A.; Greco, W.R.; Sharma, A.; Pandey, R.K.; Vaughan, L.A.; Weishaupt, K.R.; Dougherty, T.J. An in vivo quantitative structure-activity relationship for a congeneric series of pyropheophorbide derivatives as photosensitizers for photodynamic therapy. Cancer Res. 1997, 57, 4000–4007. [Google Scholar] [PubMed]
- Lobel, J.; MacDonald, I.J.; Ciesielski, M.J.; Barone, T.; Potter, W.R.; Pollina, J.; Plunkett, R.J.; Fenstermaker, R.A.; Dougherty, T.J. 2-[1-Hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) in a nude rat glioma model: Implications for photodynamic therapy. Lasers Surg. Med. 2001, 29, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Usuda, J.; Ichinose, S.; Ishizumi, T.; Hayashi, H.; Ohtani, K.; Maehara, S.; Ono, S.; Honda, H.; Kajiwara, N.; Uchida, O.; et al. Outcome of photodynamic therapy using NPe6 for bronchogenic carcinomas in central airways >1.0 cm in diameter. Clin. Cancer Res. 2010, 16, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Spikes, J.D. New trends in photobiology: Chlorins as photosensitizers in biology and medicine. J. Photochem. Photobiol. B Biol. 1190, 6, 259–274. [Google Scholar] [CrossRef]
- Smith, K.M.; Goff, D.A.; Simpson, D.J. The meso substitution of chlorophyll derivatives: Direct route for transformation of bacteriopheophorbides d into bacteriopheophorbides c. J. Am. Chem. Soc. 1985, 107, 4946–4954. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Liu, Y.; Yoon, I.; Kim, D.-K.; Yin, J.-G.; Wang, J.-J.; Shim, Y.K. Synthesis, optical properties and preliminary in vitro photodynamic effect of pyridyl and quinoxalyl substituted chlorins. Bioorg. Med. Chem. 2015, 23, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.C.; Yoon, I.; Li, J.; Lee, W.K.; Shim, Y.K. Synthesis and characterization of novel purpurinimides as photosensitizers for photodynamic therapy. Int. J. Mol. Sci. 2014, 15, 8091–8105. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.C.; Yoon, I.; Li, J.Z.; Shim, Y.K. Novel cationic purpurinimides as potential photosensitizers: Design, synthesis and biological evaluation. J. Chem. Pharm. Res. 2013, 5, 818–823. [Google Scholar]
- Li, J.Z.; Wang, J.J.; Yoon, I.; Cui, B.C.; Shim, Y.K. Synthesis of novel long wavelength cationic chlorins via stereoselective aldol-like condensation. Bioorg. Med. Chem. Lett. 2012, 22, 1846–1849. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.; Park, H.S.; Cui, B.C.; Kim, J.H.; Shim, Y.K. Synthesis and photodynamic activities of pyrazolyl and cyclopropyl derivatives of purpurin-18 methyl ester and purpurin-18-N-butylimide. Bull. Korean Chem. Soc. 2011, 32, 169–174. [Google Scholar] [CrossRef]
- Cui, B.C.; Cha, M.U.; Li, J.Z.; Park, H.S.; Yoon, I.; Shim, Y.K. Efficient synthesis and in vitro PDT effect of purpurin-18-N-aminoimides. Bull. Korean Chem. Soc. 2010, 31, 3313–3317. [Google Scholar] [CrossRef]
- Sun, B.; Li, W.; Liu, N. Curative effect of the recent photofrin photodynamic adjuvant treatment on young patients with advanced colorectal cancer. Oncol. Lett. 2016, 11, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Tobita, T.; Ohba, S.; Uehara, M.; Asahina, I. Treatment outcome of photofrin-based photodynamic therapy for T1 and T2 oral squamous cell carcinoma and dysplasia. Photodiagn. Photodyn. Ther. 2013, 10, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.M.; MacRobert, A.J.; Mosse, C.A.; Periera, B.; Bown, S.G.; Keshtgar, M.R.S. Photodynamic therapy: Inception to application in breast cancer. Breast 2017, 31, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, Y.; Zhou, L.; Zhang, L.; Lv, Y.; Li, S.; Wu, Y.; Zheng, M.; Li, W.; Gao, G.; et al. Dual-responsive molecular probe for tumor targeted imaging and photodynamic therapy. Theranostics 2017, 7, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Bazylińska, U.; Saczko, J. Nanoemulsion-templated polyelectrolyte multifunctional nanocapsules for DNA entrapment and bioimaging. Colloids Surf. B Biointerfaces 2016, 137, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Bazylińska, U.; Saczko, J.; Zielińska, K.; Wilk, K.A. Novel multilayer IR-786-loaded nanocarriers for intracellular delivering: Characterization, imaging, and internalization in human cancer cell lines. Chem. Lett. 2012, 41, 1354–1356. [Google Scholar] [CrossRef]
- Ding, Y.-F.; Li, S.; Liang, L.; Huang, Q.; Yuwen, L.; Yang, W.; Wang, R.; Wang, L.-H. Highly biocompatible chlorin e6-loaded chitosan nanoparticles for improved photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 9980–9987. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, C.; Pan, Z.; Chen, C.; Hu, D.; Gao, G.; Kang, S.; Cui, H.; Gong, P.; Cai, L. Smart hyaluronidase-actived theranostic micelles for dual-modal imaging guided photodynamic therapy. Biomaterials 2016, 101, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Kato, H.; Konaka, C.; Okunaka, T.; Ususa, J.; Ebihara, Y. Locally recurrent central-type early stage lung cancer <1.0 cm in diameter after complete remission by photodynamic therapy. Chest 2005, 128, 3269–3275. [Google Scholar]
- Seçil, M.; Çullu, N.; Aslan, G.; Mungan, U.; Uysal, F.; Tuna, B.; Yörükoğlu, K. The effect of tumor volume on survival in patients with renal cell carcinoma. Diagn. Interv. Radiol. 2012, 18, 480–487. [Google Scholar] [PubMed]
- Zhang, J.; Gold, K.A.; Lin, H.Y.; Swisher, S.G.; Xing, Y.; Lee, J.J.; Kim, E.S.; William, W.N., Jr. Relationship between tumor size and survival in non–small-cell lung cancer (NSCLC). An analysis of the surveillance, epidemiology, and end results (SEER) registry. J. Thorac. Oncol. 2015, 10, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Ball, D.; Mitchell, A.; Giroux, D.; Rami-Porta, R. Effect of tumor size on prognosis in patients treated with radical radiotherapy or chemoradiotherapy for non-small-cell lung cancer. An analysis of the staging project database of the international association for the study of lung cancer. J. Thorac. Oncol. 2013, 8, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Filonenko, E.V.; Sokolov, V.V.; Chissov, V.I.; Lukyanets, E.A.; Vorozhtsov, G.N. Photodynamic therapy of early esophageal cancer. Photodiagn. Photodyn. Ther. 2008, 5, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Usuda, J.; Kato, H.; Okunaka, T.; Furukawa, K.; Tsutsui, H.; Yamada, K.; Suga, Y.; Honda, H.; Nagatsuka, Y.; Ohira, T. Photodynamic therapy (PDT) for lung cancers. J. Thorac. Oncol. 2006, 1, 489–493. [Google Scholar] [CrossRef]
- Kofler, B.; Romani, A.; Pritz, C.; Steinbichler, T.B.; Schartinger, V.H.; Riechelmann, H.; Dudas, J. Photodynamic effect of methylene blue and low level laser radiation in head and neck squamous cell carcinoma cell lines. Int. J. Mol. Sci. 2018, 19, 1107. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Li, Z.; Zhu, S.; Zheng, Y.; Yeung, K.W.K.; Wu, S. Repeatable photodynamic therapy with triggered signaling pathways of fibroblast cell proliferation and differentiation to promote bacteria-accompanied wound healing. ACS Nano 2018, 12, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.-C.; Yang, S.-G.; Jeong, S.; Lee, D.H.; Na, K.; Kim, J.M.; Costamagna, G.; Kozarek, R.A.; Isayama, H.; Deviere, J.; et al. Polymeric photosensitizer-embedded self-expanding metal stent for repeatable endoscopic photodynamic therapy of cholangiocarcinoma. Biomaterials 2014, 35, 8487–8495. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-E.; Yoon, I.-S.; Sun, P.-L.; Yi, E.; Jheon, S.; Shim, C.K. Anticancer efficacy of photodynamic therapy with hematoporphyrin-modified, doxorubicin-loaded nanoparticles in liver cancer. J. Photochem. Photobiol. B Biol. 2014, 140, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-E.; Cho, H.-J.; Yi, E.; Kim, D.D.; Jheon, S. Hypocrellin B and and paclitaxel-encapsulated hyaluronic acid–ceramide nanoparticles for targeted photodynamic therapy in lung cancer. J. Photochem. Photobiol. B Biol. 2016, 158, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, T.; Xing, D.; Wang, F.; Pei, Y.; Wei, X. Single cell analysis of PKC activation during proliferation and apoptosis induced by laser irradiation. J. Cell. Physiol. 2006, 206, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Koo, H.; Jeong, H.; Huh, M.S.; Choi, Y.; Jeong, S.Y.; Byun, Y.; Choi, K.; Kim, K.; Kwon, I.C. Comparative study of photosensitizer loaded and conjugated glycol chitosan nanoparticles for cancer therapy. J. Control. Release 2011, 152, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Xu, L.; Xue, Y.; Jiang, X.; Zhang, W. Enhancing photochemical internalization of DOX through a porphyrin-based amphiphilic block copolymer. Biomacromolecules 2017, 18, 3992–4001. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cheng, A.-C.; Wang, M.-S.; Peng, X. Detection of apoptosis induced by new type gosling viral enteritis virus in vitro through fluorescein annexin V-FITC/PI double labeling. World J. Gastroenterol. 2008, 14, 2174–2178. [Google Scholar] [CrossRef] [PubMed]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar] [CrossRef] [PubMed]












| Cell Line (Assay) | Incubation Time (h) | MPPa (µM) | NMPi (µM) |
|---|---|---|---|
| A549 a (MTT) | 3 | 0.89 | 1.15 |
| 24 | 0.44 | 0.73 | |
| HeLa b (WST−8) | 12 | 0.28 | 2.71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.-E.; Liu, Y.; Lee, T.H.; Lee, W.K.; Yoon, I.; Kim, K. Tumor Size-Dependent Anticancer Efficacy of Chlorin Derivatives for Photodynamic Therapy. Int. J. Mol. Sci. 2018, 19, 1596. https://doi.org/10.3390/ijms19061596
Chang J-E, Liu Y, Lee TH, Lee WK, Yoon I, Kim K. Tumor Size-Dependent Anticancer Efficacy of Chlorin Derivatives for Photodynamic Therapy. International Journal of Molecular Sciences. 2018; 19(6):1596. https://doi.org/10.3390/ijms19061596
Chicago/Turabian StyleChang, Ji-Eun, Yang Liu, Tae Heon Lee, Woo Kyoung Lee, Il Yoon, and Kwhanmien Kim. 2018. "Tumor Size-Dependent Anticancer Efficacy of Chlorin Derivatives for Photodynamic Therapy" International Journal of Molecular Sciences 19, no. 6: 1596. https://doi.org/10.3390/ijms19061596
APA StyleChang, J. -E., Liu, Y., Lee, T. H., Lee, W. K., Yoon, I., & Kim, K. (2018). Tumor Size-Dependent Anticancer Efficacy of Chlorin Derivatives for Photodynamic Therapy. International Journal of Molecular Sciences, 19(6), 1596. https://doi.org/10.3390/ijms19061596