Circulating Cell-Free DNA as a Prognostic and Molecular Marker for Patients with Brain Tumors under Perillyl Alcohol-Based Therapy
Abstract
:1. Introduction
2. Results
2.1. Detection of Circulating cfDNA in Serum of Patients with Brain Tumors
2.2. Diagnostic Power of cfDNA Serum Levels for Brain Tumor Evaluation
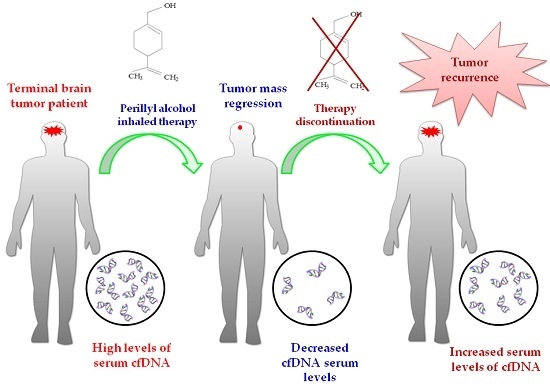
2.3. Influence of POH-Based Therapy upon cfDNA Levels and Patient Survival
2.4. Effect of POH-Based Therapy on cfDNA Levels and the Disease Process
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Sample Collection and DNA Isolation
4.3. Drug Administration and Dose Escalation
4.4. Patient Evaluation
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BBB | Blood brain barrier |
| BM | Brain metastasis |
| cfDNA | Circulating cell-free DNA |
| CNA | Circulating nucleic acids |
| CSF | Cerebrospinal fluid |
| GBM | Glioblastoma |
| IDH | Isocitrate dehydrogenase |
| ITN | Intranasal |
| MGMT | O6-Methylguanine DNA methyltransferase |
| MRI | Magnetic resonance image |
| PET | Positron emission tomography |
| POH | Perillyl alcohol |
| ROC | Receiver operating characteristic |
| TMZ | Temozolomide |
| WHO | World Health Organization |
References
- Langley, R.R.; Fidler, I.J. The biology of brain metastasis. Clin. Chem. 2013, 59, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Kwak, H.-J.; Lee, S.-H. Role of hyaluronan in glioma invasion. Cell Adhes. Migr. 2008, 2, 202–207. [Google Scholar] [CrossRef]
- Carlsson, S.K.; Brothers, S.P.; Wahlestedt, C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol. Med. 2014, 6, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- La Fougère, C.; Suchorska, B.; Bartenstein, P.; Kreth, F.-W.; Tonn, J.-C. Molecular imaging of gliomas with PET: Opportunities and limitations. Neuro-oncology 2011, 13, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Egger, J.; Kapur, T.; Fedorov, A.; Pieper, S.; Miller, J.V.; Veeraraghavan, H.; Freisleben, B.; Golby, A.J.; Nimsky, C.; Kikinis, R. GBM volumetry using the 3D Slicer medical image computing platform. Sci. Rep. 2013, 3, 1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takenaka, S.; Asano, Y.; Shinoda, J.; Nomura, Y.; Yonezawa, S.; Miwa, K.; Yano, H.; Iwama, T. Comparison of 11C-methionine, 11C-choline, and 18F-fluorodeoxyglucose-PET for distinguishing glioma recurrence from radiation necrosis. Neurol. Med. Chir. 2014, 54, 280–289. [Google Scholar] [CrossRef]
- Grech-Sollars, M.; Ordidge, K.; Honeyfield, L.; Vaqas, B.; Khan, S.; O’Neil, K.; Peterson, D.; Roncaroli, F.; Towey, D.; Barwick, T. Nimg-3118f-methylcholine PET/CT and magnetic resonance spectroscopy imaging and tissue biomarkers of cell membrane turnover in primary brain gliomas—A pilot study. Neuro-oncology 2015, 17, v160. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Persano, L.; Rampazzo, E.; Basso, G.; Viola, G. Glioblastoma cancer stem cells: Role of the microenvironment and therapeutic targeting. Biochem. Pharmacol. 2013, 85, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Stroun, M.; Maurice, P.; Vasioukhin, V.; Lyautey, J.; Lederrey, C.; Lefort, F.; Rossier, A.; Chen, X.Q.; Anker, P. The origin and mechanism of circulating DNA. Ann. N. Y. Acad. Sci. 2000, 906, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gahan, P.B.; Swaminathan, R. Circulating nucleic acids in plasma and serum. Ann. N. Y. Acad. Sci. 2008, 1137, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stroun, M.; Anker, P.; Lyautey, J.; Lederrey, C.; Maurice, P.A. Isolation and characterization of DNA from the plasma of cancer patients. Eur. J. Cancer Clin. Oncol. 1987, 23, 707–712. [Google Scholar] [CrossRef]
- Stroun, M.; Anker, P.; Maurice, P.; Lyautey, J.; Lederrey, C.; Beljanski, M. Neoplastic characteristics of the DNA found in the plasma of cancer patients. Oncology 1989, 46, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Aarthy, R.; Mani, S.; Velusami, S.; Sundarsingh, S.; Rajkumar, T. Role of Circulating Cell-Free DNA in Cancers. Mol. Diagn. Ther. 2015, 19, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Jahr, S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.O.; Hesch, R.-D.; Knippers, R. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001, 61, 1659–1665. [Google Scholar] [PubMed]
- Lin, Z.; Neiswender, J.; Fang, B.; Ma, X.; Zhang, J.; Hu, X. Value of circulating cell-free DNA analysis as a diagnostic tool for breast cancer: A meta-analysis. Oncotarget 2017, 8, 26625–26636. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, C.O.; Teixeira, R.M.; Silva, J.C.T.; Fischer, J.D.S.D.G.; Meirelles, O.C.; Landeiro, J.A.; Quirico-Santos, T. Long-term outcome in patients with recurrent malignant glioma treated with Perillyl alcohol inhalation. Anticancer Res. 2013, 33, 5625–5631. [Google Scholar] [PubMed]
- Da Fonseca, C.O.; Simao, M.; Lins, I.R.; Caetano, R.O.; Futuro, D.; Quirico-Santos, T. Efficacy of monoterpene perillyl alcohol upon survival rate of patients with recurrent glioblastoma. J. Cancer Res. Clin. Oncol. 2011, 137, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Mello, A.L.; Ribeiro, M.G.; Garcia, D.G.; Da Fonseca, C.O.; Salazar, M.D.A.; Schönthal, A.H.; Quirico-Santos, T. Perillyl alcohol, a pleiotropic natural compound suitable for brain tumor therapy, targets free radicals. Arch. Immunol. Ther. Exp. 2017, 65, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Springer, S.; Zhang, M.; McMahon, K.W.; Kinde, I.; Dobbyn, L.; Ptak, J.; Brem, H.; Chaichana, K.; Gallia, G.L. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc. Natl. Acad. Sci. USA 2015, 112, 9704–9709. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Pantel, K.; Müller, V.; Rack, B.; Kasimir-Bauer, S.; Janni, W.; Schwarzenbach, H. Apoptosis-related deregulation of proteolytic activities and high serum levels of circulating nucleosomes and DNA in blood correlate with breast cancer progression. BMC Cancer 2011, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Tamkovich, S.N.; Cherepanova, A.V.; Kolesnikova, E.V.; Rykova, E.Y.; Pyshnyi, D.V.; Vlassov, V.V.; Laktionov, P.P. Circulating DNA and DNase activity in human blood. Ann. N. Y. Acad. Sci. 2006, 1075, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Tamkovich, S.N.; Litviakov, N.V.; Bryzgunova, O.E.; Dobrodeev, A.Y.; Rykova, E.Y.; Tuzikov, S.A.; Zav’Ialov, A.A.; Vlassov, V.V.; Cherdyntseva, N.V.; Laktionov, P.P. Cell-Surface-Bound Circulating DNA as a Prognostic Factor in Lung Cancer. Ann. N. Y. Acad. Sci. 2008, 1137, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The biology of brain metastasis: Challenges for therapy. Cancer J. 2015, 21, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Fleischhacker, M.; Schmidt, B. Circulating nucleic acids (CNAs) and cancer—A survey. Biochim. Biophys. Acta 2007, 1775, 181–232. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-L.; Kim, H.J.; Choi, B.Y.; Lee, H.-C.; Jang, H.-R.; Song, K.S.; Noh, S.-M.; Kim, S.-Y.; Han, D.S.; Kim, Y.S. Quantitative analysis of cell-free DNA in the plasma of gastric cancer patients. Oncol. Lett. 2012, 3, 921–926. [Google Scholar] [PubMed]
- Herrera, L.J.; Raja, S.; Gooding, W.E.; El-Hefnawy, T.; Kelly, L.; Luketich, J.D.; Godfrey, T.E. Quantitative analysis of circulating plasma DNA as a tumor marker in thoracic malignancies. Clin. Chem. 2005, 51, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, T.; Berger, A.; Zinzindohoué, F.; Micard, S.; Landi, B.; Blons, H.; Beaune, P.; Cugnenc, P.H.; Laurent-Puig, P. Detection of free-circulating tumor-associated DNA in plasma of colorectal cancer patients and its association with prognosis. Int. J. Cancer 2002, 100, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Maire, F.; Micard, S.; Hammel, P.; Voitot, H.; Lévy, P.; Cugnenc, P.; Ruszniewski, P.; Puig, P.L. Differential diagnosis between chronic pancreatitis and pancreatic cancer: Value of the detection of KRAS2 mutations in circulating DNA. Br. J. Cancer 2002, 87, 551. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, G.; Conte, D.; Leon, M.; Cirincione, R.; Roz, L.; Ratcliffe, C.; Roz, E.; Cirenei, N.; Bellomi, M.; Pelosi, G.; et al. Quantification of Free Circulating DNA As a Diagnostic Marker in Lung Cancer. J. Clin. Oncol. 2003, 21, 3902–3908. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Lv, C.; Qi, J.; Zhao, W.; Wu, X.; Jing, R.; Wu, X.; Ju, S.; Chen, J. Prognostic value of free DNA quantification in serum and cerebrospinal fluid in glioma patients. J. Mol. Neurosci. 2012, 46, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.W.; Ludwig, T.; Tatenhorst, L.; Braune, S.; Oberleithner, H.; Senner, V.; Paulus, W. Glioblastoma cells release factors that disrupt blood-brain barrier features. Acta Neuropathol. 2004, 107, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Stephan, C.; Lewandowski, M.; Klotzek, S.; Jung, M.; Kristiansen, G.; Lein, M.; Loening, S.A.; Schnorr, D. Increased cell-free DNA in plasma of patients with metastatic spread in prostate cancer. Cancer Lett. 2004, 205, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A., Jr.; Williams, R.T.; Wu, J.; Kinde, I.; Hecht, J.R.; Berlin, J.; Allen, B.; Bozic, I.; Reiter, J.G.; Nowak, M.A. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012, 486, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bettegowda, C. Applications of DNA-based liquid biopsy for central nervous system neoplasms. J. Mol. Diagn. 2017, 19, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.Y.-L.; Rainer, T.H.; Wong, L.K.-S.; Lam, W.; Lo, Y.-M.D. Plasma DNA as a prognostic marker for stroke patients with negative neuroimaging within the first 24 h of symptom onset. Resuscitation 2006, 68, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Cabral, R.; Neto, J.; Carvalho, M. Circulating DNA as a biomarker for early detection of cancer: A brief update with an emphasis on lung cancer. Open Lung Cancer J. 2010, 3, 38–44. [Google Scholar] [CrossRef]
- Santos, J.G.; Da Cruz, W.M.S.; Schönthal, A.H.; Salazar, M.; Fontes, C.A.P.; Quirico-Santos, T.; Da Fonseca, C.O. Efficacy of a ketogenic diet with concomitant intranasal perillyl alcohol as a novel strategy for the therapy of recurrent glioblastoma. Oncol. Lett. 2018, 15, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Obuchowski, N.A.; Lieber, M.L.; Wians, F.H. ROC curves in clinical chemistry: Uses, misuses, and possible solutions. Clin. Chem. 2004, 50, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.V.; Pan, J.; Rai, S.N.; Galandiuk, S. ROC-ing along: Evaluation and interpretation of receiver operating characteristic curves. Surgery 2016, 159, 1638–1645. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, G.; Silva, E.; Da Fonseca, C.; Quirico-Santos, T. Circulating Cell-Free DNA as a Prognostic and Molecular Marker for Patients with Brain Tumors under Perillyl Alcohol-Based Therapy. Int. J. Mol. Sci. 2018, 19, 1610. https://doi.org/10.3390/ijms19061610
Faria G, Silva E, Da Fonseca C, Quirico-Santos T. Circulating Cell-Free DNA as a Prognostic and Molecular Marker for Patients with Brain Tumors under Perillyl Alcohol-Based Therapy. International Journal of Molecular Sciences. 2018; 19(6):1610. https://doi.org/10.3390/ijms19061610
Chicago/Turabian StyleFaria, Giselle, Emanuelle Silva, Clovis Da Fonseca, and Thereza Quirico-Santos. 2018. "Circulating Cell-Free DNA as a Prognostic and Molecular Marker for Patients with Brain Tumors under Perillyl Alcohol-Based Therapy" International Journal of Molecular Sciences 19, no. 6: 1610. https://doi.org/10.3390/ijms19061610
APA StyleFaria, G., Silva, E., Da Fonseca, C., & Quirico-Santos, T. (2018). Circulating Cell-Free DNA as a Prognostic and Molecular Marker for Patients with Brain Tumors under Perillyl Alcohol-Based Therapy. International Journal of Molecular Sciences, 19(6), 1610. https://doi.org/10.3390/ijms19061610