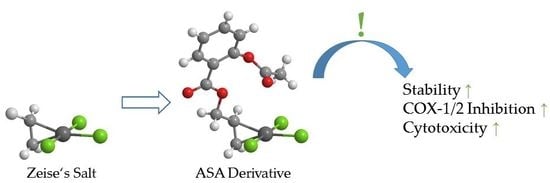
Synthesis and Biological Evaluation of Zeise’s Salt Derivatives with Acetylsalicylic Acid Substructure
Abstract
:1. Introduction
2. Results
2.1. Syntheses and Characterization
2.1.1. Syntheses
2.1.2. X-ray Structure Analysis
2.1.3. Molecular Characterization by NMR Spectroscopy
2.1.4. Characterization by Mass Spectrometry
2.2. Evaluation of Stability
2.3. Biological Evaluation
2.3.1. COX-1/2 Isoenzyme Inhibition
2.3.2. Antiproliferative Effects
3. Discussion
4. Materials and Methods
4.1. General Aspects
4.2. Capillary Electrophoresis
4.3. X-ray Crystallography
4.4. General Procedure for the Synthesis of the Acetylsalicylic Acid Esters 1–4
4.4.1. (Prop-2-en-1-yl)-2-acetoxybenzoate (Propene-ASA, 1)
4.4.2. (But-3-en-1-yl)-2-acetoxybenzoate (Butene-ASA, 2)
4.4.3. (Pent-4-en-1-yl)-2-acetoxybenzoate (Pentene-ASA, 3)
4.4.4. (Hex-5-en-1-yl)-2-acetoxybenzoate (Hexene-ASA, 4)
4.5. General Procedure for the Synthesis of the Zeise’s Salt Derivatives 1a–4a
4.5.1. Potassium {trichlorido[η2-(prop-2-en-1-yl)-2-acetoxybenzoate]platinate(II)} (Pt-Propene-ASA, 1a)
4.5.2. Potassium {trichlorido[η2-(but-3-en-1-yl)-2-acetoxybenzoate]platinate(II)} (Pt-Butene-ASA, 2a)
4.5.3. Potassium {trichlorido[η2-(pent-4-en-1-yl)-2-acetoxybenzoate]platinate(II)} (Pt-Pentene-ASA, 3a)
4.5.4. Potassium {trichlorido[η2-(hex-5-en-1-yl)-2-acetoxybenzoate]platinate(II)} (Pt-Hexene-ASA, 4a)
4.6. General Methods for the Cell Culture
4.6.1. COX-1/2 Isoenzyme Inhibition
4.6.2. Antiproliferative Effects
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA | acetylsalicylic acid |
| BGE | background electrolyte |
| CCDC | Cambridge Crystallographic Data Centre |
| CE | capillary electrophoresis |
| COX | cyclooxygenase |
| DAD | diode array detector |
| DCC | N,N′-dicyclohexylcarbodiimide |
| DMAP | 4-dimethylaminopyridine |
| DMF | dimethylformamide |
| DMSO | dimethyl sulfoxide |
| HR-ESI-MS | high resolution electrospray ionization mass spectrometry |
| m.p. | melting point |
| NMR | nuclear magnetic resonance |
| NSAID | nonsteroidal anti-inflammatory drug |
| PBS | phosphate-buffered saline |
| SA | salicylic acid |
| TLC | thin layer chromatography |
| TMS | tetramethylsilane |
| τ1/2 | half-live |
References
- Peyrone, M. Ueber die Einwirkung des Ammoniaks auf Platinchlorür. Eur. J. Org. Chem. 1844, 51, 1–29. [Google Scholar] [CrossRef]
- Rosenberg, B.; van Camp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.V.; Hambley, T.W. Platinum drug distribution in cancer cells and tumors. Chem. Rev. 2009, 109, 4911–4920. [Google Scholar] [CrossRef] [PubMed]
- Meier-Menches, S.M.; Gerner, C.; Berger, W.; Hartinger, C.G.; Keppler, B.K. Structure–activity relationships for ruthenium and osmium anticancer agents—Towards clinical development. Chem. Soc. Rev. 2018, 47, 909–928. [Google Scholar] [CrossRef] [PubMed]
- Aman, F.; Hanif, M.; Kubanik, M.; Ashraf, A.; Söhnel, T.; Jamieson, S.M.; Siddiqui, W.A.; Hartinger, C.G. Anti-inflammatory oxicams as multi-donor ligand systems: PH-and solvent-dependent coordination modes of meloxicam and piroxicam to Ru and Os. Chem. Eur. J. 2017, 23, 4893–4902. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Hanif, M.; Kubanik, M.; Söhnel, T.; Jamieson, S.M.; Bhattacharyya, A.; Hartinger, C.G. Aspirin-inspired organometallic compounds: Structural characterization and cytotoxicity. J. Organomet. Chem. 2017, 839, 31–37. [Google Scholar] [CrossRef]
- Aman, F.; Hanif, M.; Siddiqui, W.A.; Ashraf, A.; Filak, L.K.; Reynisson, J.H.; Söhnel, T.; Jamieson, S.M.; Hartinger, C.G. Anticancer ruthenium (η6-p-cymene) complexes of nonsteroidal anti-inflammatory drug derivatives. Organometallics 2014, 33, 5546–5553. [Google Scholar] [CrossRef]
- Pathak, R.K.; Marrache, S.; Choi, J.H.; Berding, T.B.; Dhar, S. The prodrug platin-A: Simultaneous release of cisplatin and aspirin. Angew. Chem. Int. Ed. 2014, 126, 1994–1998. [Google Scholar] [CrossRef]
- Ang, W.H.; Casini, A.; Sava, G.; Dyson, P.J. Organometallic ruthenium-based antitumor compounds with novel modes of action. J. Organomet. Chem. 2011, 696, 989–998. [Google Scholar] [CrossRef]
- Rubner, G.; Bensdorf, K.; Wellner, A.; Bergemann, S.; Gust, R. Synthesis, characterisation and biological evaluation of copper and silver complexes based on acetylsalicylic acid. Arch. Pharm. Chem. Life Sci. 2011, 344, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Rubner, G.; Bensdorf, K.; Wellner, A.; Kircher, B.; Bergemann, S.; Ott, I.; Gust, R. Synthesis and biological activities of transition metal complexes based on acetylsalicylic acid as neo-anticancer agents. J. Med. Chem. 2010, 53, 6889–6898. [Google Scholar] [CrossRef] [PubMed]
- Rubner, G.; Bensdorf, K.; Wellner, A.; Bergemann, S.; Ott, I.; Gust, R. [Cyclopentadienyl]metalcarbonyl complexes of acetylsalicylic acid as neo-anticancer agents. Eur. J. Med. Chem. 2010, 45, 5157–5163. [Google Scholar] [CrossRef] [PubMed]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Ott, I.; Gust, R. Non platinum metal complexes as anti-cancer drugs. Arch. Pharm. Chem. Life Sci. 2007, 340, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Ott, I.; Schmidt, K.; Kircher, B.; Schumacher, P.; Wiglenda, T.; Gust, R. Antitumor-active cobalt−alkyne complexes derived from acetylsalicylic acid: Studies on the mode of drug action. J. Med. Chem. 2005, 48, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Winzer, K.-J.; Müller, B.-M.; Weichert, W.; Pest, S.; Köbel, M.; Kristiansen, G.; Reles, A.; Siegert, A.; Guski, H.; et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer 2003, 97, 2978–2987. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, G.; Denkert, C.; Schlüns, K.; Dahl, E.; Pilarsky, C.; Hauptmann, S. CD24 is expressed in ovarian cancer and is a new independent prognostic marker of patient survival. Am. J. Pathol. 2002, 161, 1215–1221. [Google Scholar] [CrossRef]
- Meieranz, S.; Stefanopoulou, M.; Rubner, G.; Bensdorf, K.; Kubutat, D.; Sheldrick, W.S.; Gust, R. The biological activity of Zeise’s salt and its derivatives. Angew. Chem. Int. Ed. 2015, 54, 2834–2837. [Google Scholar] [CrossRef] [PubMed]
- Neises, B.; Steglich, W. Simple method for the esterification of carboxylic acids. Angew. Chem. Int. Ed. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Love, R.A.; Koetzle, T.F.; Williams, G.J.B.; Andrews, L.C.; Bau, R. Neutron diffraction study of the structure of Zeise’s salt, KPtCl3(C2H4)*H2O. Inorg. Chem. 1975, 14, 2653–2657. [Google Scholar] [CrossRef]
- Shamsuddin, S.; Santillan, C.C.; Stark, J.L.; Whitmire, K.H.; Siddik, Z.H.; Khokhar, A.R. Synthesis, characterization, and antitumor activity of new platinum(IV) trans-carboxylate complexes: Crystal structure of [Pt(cis-1,4-DACH)trans-(acetate)2Cl2]. J. Inorg. Biochem. 1998, 71, 29–35. [Google Scholar] [CrossRef]
- Joy, J.R.; Orchin, M. Hydrolyse des Zeise-Salzes. Z. anorg. allg. Chem. 1960, 305, 236–240. [Google Scholar] [CrossRef]
- Obermoser, V.; Urban, M.E.; Murgueitio, M.S.; Wolber, G.; Kintscher, U.; Gust, R. New telmisartan-derived PPARγ agonists: Impact of the 3D-binding mode on the pharmacological profile. Eur. J. Med. Chem. 2016, 124, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Nishihara, R.; Wu, K.; Wang, M.; Ogino, S.; Willett, W.C.; Spiegelman, D.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016, 2, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Gerdes, A.-M.; Macrae, F.; Mecklin, J.-P.; Moeslein, G.; Olschwang, S.; Eccles, D.; Evans, D.G.; Maher, E.R.; Bertario, L.; et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: An analysis from the CAPP2 randomised controlled trial. Lancet 2011, 378, 2081–2087. [Google Scholar] [CrossRef]
- Miao, R.; Yang, G.; Miao, Y.; Mei, Y.; Hong, J.; Zhao, C.; Zhu, L. Interactions of platinum(II) complexes with sulfur-containing peptides studied by electrospray ionization mass spectrometry and tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Reedijk, J. Why does cisplatin reach guanine-N7 with competing S-donor ligands available in the cell? Chem. Rev. 1999, 99, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, G.R.; Miller, A.J.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-2013/1 Program Suite for the Solution and Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 2013. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]






| Structural Element | Bond Length/Å | Bond Angle/° |
|---|---|---|
| Pt(1)–C(1) | 2.114(3) | |
| Pt(1)–C(2) | 2.155(3) | |
| Pt(1)–Cl(3) (cis) | 2.3030(7) | |
| Pt(1)–Cl(2) (cis) | 2.3033(7) | |
| Pt(1)–Cl(1) (trans) | 2.3244(7) | |
| C(1)–C(2) | 1.405(4) | |
| C(2)–C(3) | 1.484(4) | |
| C(1)–C(2)–C(3) | 119.0(3) | |
| C(1)–C(2)–H(2) | 120.0(2) | |
| C(3)–C(2)–H(2) | 115.0(2) | |
| Pt(1)–C(2)–C(1) | 69.2(2) | |
| Pt(1)–C(2)–C(3) | 119.5(2) | |
| Pt(1)–C(2)–H(2) | 104.6(2) |
| Compound | 1H NMR | 13C NMR | ||||||
|---|---|---|---|---|---|---|---|---|
| –CH=CH2 | Δδ | –CH=CH2 | Δδ | –CH=CH2 | Δδ | –CH=CH2 | Δδ | |
| 1 | 6.06 | 1.06 | 5.42/5.28 | 1.08/0.97 | 133.43 | 55.87 | 118.65 | 53.55 |
| 1a | 5.00 | 4.34/4.31 | 77.56 | 65.10 | ||||
| 2 | 5.90 | 0.88 | 5.18/5.08 | 0.93/0.83 | 135.26 | 51.08 | 117.59 | 51.63 |
| 2a | 5.02 | 4.25 | 84.18 | 65.96 | ||||
| 3 | 5.88 | 0.88 | 5.08/4.99 | 0.91/0.82 | 138.59 | 49.68 | 115.59 | 50.54 |
| 3a | 5.00 | 4.17 | 88.91 | 65.05 | ||||
| 4 | 5.84 | 0.87 | 5.04/4.95 | 0.89/0.80 | 139.33 | 49.73 | 115.18 | 50.34 |
| 4a | 4.97 | 4.15 | 89.60 | 64.84 | ||||
| Compound | IC50/µM 1 | |
|---|---|---|
| HT-29 | MCF-7 | |
| ASA | ≥50 | ≥50 |
| Zeise’s salt | ≥50 | ≥50 |
| 1a | 49.7 ± 1.8 | ≥50 |
| 2a | 31.4 ± 0.4 | 30.1 ± 1.5 |
| 3a | 44.2 ± 3.4 | 37.4 ± 2.5 |
| 4a | 41.4 ± 0.3 | 43.7 ± 5.7 |
| Cisplatin | 2.6 ± 0.1 | 3.7 ± 0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weninger, A.; Baecker, D.; Obermoser, V.; Egger, D.; Wurst, K.; Gust, R. Synthesis and Biological Evaluation of Zeise’s Salt Derivatives with Acetylsalicylic Acid Substructure. Int. J. Mol. Sci. 2018, 19, 1612. https://doi.org/10.3390/ijms19061612
Weninger A, Baecker D, Obermoser V, Egger D, Wurst K, Gust R. Synthesis and Biological Evaluation of Zeise’s Salt Derivatives with Acetylsalicylic Acid Substructure. International Journal of Molecular Sciences. 2018; 19(6):1612. https://doi.org/10.3390/ijms19061612
Chicago/Turabian StyleWeninger, Alexander, Daniel Baecker, Victoria Obermoser, Dorothea Egger, Klaus Wurst, and Ronald Gust. 2018. "Synthesis and Biological Evaluation of Zeise’s Salt Derivatives with Acetylsalicylic Acid Substructure" International Journal of Molecular Sciences 19, no. 6: 1612. https://doi.org/10.3390/ijms19061612
APA StyleWeninger, A., Baecker, D., Obermoser, V., Egger, D., Wurst, K., & Gust, R. (2018). Synthesis and Biological Evaluation of Zeise’s Salt Derivatives with Acetylsalicylic Acid Substructure. International Journal of Molecular Sciences, 19(6), 1612. https://doi.org/10.3390/ijms19061612