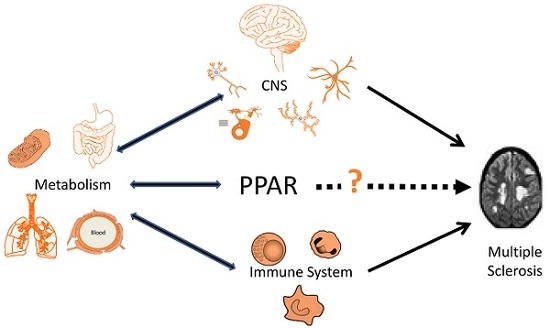
Metabolic Dysfunction and Peroxisome Proliferator-Activated Receptors (PPAR) in Multiple Sclerosis
Abstract
:1. An Overview of Multiple Sclerosis (MS)
2. Genes and the Environment in MS
3. Genesis and Repair of MS Lesions
3.1. Demyelination Lesions
3.2. Neurodegeneration and Progressive MS
4. Systemic Metabolism in MS
4.1. Plasma Lipids
4.2. Metabolism and Immune Dysfunction
5. Peroxisome Proliferator-Activated Receptors (PPAR) in MS
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, M.K.; Yong, V.W. Myeloid cells—Targets of medication in multiple sclerosis. Nat. Rev. Neurol. 2016, 12, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Metz, I.; Weigand, S.D.; Popescu, B.F.G.; Frischer, J.M.; Parisi, J.E.; Guo, Y.; Lassmann, H.; Brück, W.; Lucchinetti, C.F. Pathologic heterogeneity persists in early active multiple sclerosis lesions. Ann. Neurol. 2014, 75, 728–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L.; Zamvil, S.S. Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol. 2005, 26, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.; Wimmer, I.; Hametner, S.; Haider, L.; Van Dam, A.-M.; Liblau, R.S.; Smith, K.J.; Probert, L.; Binder, C.J.; Bauer, J.; et al. Oxidative tissue injury in multiple sclerosis is only partly reflected in experimental disease models. Acta Neuropathol. 2014, 128, 247–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrewe, L.; Lill, C.M.; Liu, T.; Salmen, A.; Gerdes, L.A.; Guillot-Noel, L.; Akkad, D.A.; Blaschke, P.; Graetz, C.; Hoffjan, S.; et al. Investigation of sex-specific effects of apolipoprotein E on severity of EAE and MS. J. Neuroinflamm. 2015, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Cohen, J.A.; Coles, A.J.; Hartung, H.-P.; Havrdova, E.; Selmaj, K.W.; Margolin, D.H.; Lake, S.L.; Kaup, S.M.; Panzara, M.A.; et al. CARE-MS II Investigators Alemtuzumab improves preexisting disability in active relapsing-remitting MS patients. Neurology 2016, 87, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Lorscheider, J.; Jokubaitis, V.G.; Spelman, T.; Izquierdo, G.; Lugaresi, A.; Havrdova, E.; Horakova, D.; Trojano, M.; Duquette, P.; Girard, M.; et al. MSBase Study Group Anti-inflammatory disease-modifying treatment and short-term disability progression in SPMS. Neurology 2017, 89, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Westad, A.; Venugopal, A.; Snyder, E. The multiple sclerosis market. Nat. Rev. Drug Discov. 2017, 16, 675–676. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Vukusic, S. Natural history of multiple sclerosis: A unifying concept. Brain 2006, 129, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Weiner, H.L. The challenge of multiple sclerosis: How do we cure a chronic heterogeneous disease? Ann. Neurol. 2009, 65, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapp, B.D.; Nave, K.-A. Multiple Sclerosis: An Immune or Neurodegenerative Disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, M.; Magliozzi, R.; Ciccarelli, O.; Geurts, J.J.G.; Reynolds, R.; Martin, R. Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 2015, 16, 147–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.J.; Tanasescu, R.; Gran, B. Innate immune regulation of autoimmunity in multiple sclerosis: Focus on the role of Toll-like receptor 2. J. Neuroimmunol. 2017, 304, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wolfgram, F. What if multiple sclerosis isn’t an immunological or a viral disease? The case for a circulating toxin. Neurochem. Res. 1979, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mastronardi, F.G.; Moscarello, M.A. Molecules affecting myelin stability: A novel hypothesis regarding the pathogenesis of multiple sclerosis. J. Neurosci. Res. 2005, 80, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.H.; Henderson, A.P.D.; Prineas, J.W. The macrophage in MS: Just a scavenger after all? Pathology and pathogenesis of the acute MS lesion. Mult. Scler. 2006, 12, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Stys, P.K.; Zamponi, G.W.; Van Minnen, J.; Geurts, J.J.G. Will the real multiple sclerosis please stand up? Nat. Rev. Neurosci. 2012, 13, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, I.A.; Mehler, M.F. Towards a “systems”-level understanding of the nervous system and its disorders. Trends Neurosci. 2013, 36, 674–684. [Google Scholar] [CrossRef] [PubMed]
- McMurran, C.E.; Jones, C.A.; Fitzgerald, D.C.; Franklin, R.J.M. CNS Remyelination and the Innate Immune System. Front. Cell Dev. Biol. 2016, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, Y.; O’Hagan, T.; Dittmer, M.; Penalva, R.; Mayoral, S.R.; Bankhead, P.; Fleville, S.; Eleftheriadis, G.; Zhao, C.; Naughton, M.; et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 2017, 20, 674–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Bos, S.D.; Harbo, H.F.; Thompson, W.K.; Schork, A.J.; Bettella, F.; Witoelar, A.; Lie, B.A.; Li, W.; McEvoy, L.K.; et al. Genetic overlap between multiple sclerosis and several cardiovascular disease risk factors. Mult. Scler. J. 2016, 22, 1783–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokry, L.E.; Ross, S.; Timpson, N.J.; Sawcer, S.; Davey Smith, G.; Richards, J.B. Obesity and Multiple Sclerosis: A Mendelian Randomization Study. PLoS Med. 2016, 13, e1002053. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, C.; Lucas, R.; Taylor, B. Environmental Risk Factors for Multiple Sclerosis: A Review with a Focus on Molecular Mechanisms. Int. J. Mol. Sci. 2012, 13, 11718–11752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2016, 13, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Sena, A.; Couderc, R.; Ferret-Sena, V.; Pedrosa, R.; Andrade, M.L.; Araujo, C.; Roque, R.; Cascais, M.J.; Morais, M.G. Apolipoprotein E polymorphism interacts with cigarette smoking in progression of multiple sclerosis. Eur. J. Neurol. 2009, 16, 832–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munger, K.L.; Fitzgerald, K.C.; Freedman, M.S.; Hartung, H.-P.; Miller, D.H.; Montalbán, X.; Edan, G.; Barkhof, F.; Suarez, G.; Radue, E.-W.; et al. No association of multiple sclerosis activity and progression with EBV or tobacco use in BENEFIT. Neurology 2015, 85, 1694–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese, M.; Yuan, C.; Chitnis, T.; Ascherio, A.; Munger, K.L. No association between dietary sodium intake and the risk of multiple sclerosis. Neurology 2017, 89, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Mokry, L.E.; Ross, S.; Ahmad, O.S.; Forgetta, V.; Smith, G.D.; Leong, A.; Greenwood, C.M.T.; Thanassoulis, G.; Richards, J.B. Vitamin D and Risk of Multiple Sclerosis: A Mendelian Randomization Study. PLoS Med. 2015, 12, e1001866. [Google Scholar] [CrossRef] [PubMed]
- Goodin, D.S. The nature of genetic susceptibility to multiple sclerosis: Constraining the possibilities. BMC Neurol. 2016, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H. A biochemical approach to the problem of multiple sclerosis. Proc. R. Soc. Med. 1966, 59, 269–276. [Google Scholar] [PubMed]
- Goldberg, P. Multiple sclerosis: Vitamin D and calcium as environmental determinants of prevalence. (A Viewpoint) Part I: Sunlight, Dietary Factors and Epidemiology. Intern. J. Environ. Stud. 1974, 6, 19–27. [Google Scholar] [CrossRef]
- Goldberg, P. Multiple sclerosis: Vitamin D and calcium as environmental determinants of prevalence. (A viewpoint). Part II. Biochemical and genetic factores. Intern. J. Environ. Stud. 1974, 6, 121–129. [Google Scholar] [CrossRef]
- Swank, R.L. Multiple sclerosis: Twenty years on low fat diet. Arch. Neurol. 1970, 23, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Alter, M.; Yamoor, M.; Harshe, M. Multiple sclerosis and nutrition. Arch. Neurol. 1974, 31, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Agranoff, B.W.; Goldberg, D. Diet and the geographical distribution of multiple sclerosis. Lancet 1974, 2, 1061–1066. [Google Scholar] [CrossRef]
- Jelinek, G.A.; De Livera, A.M.; Marck, C.H.; Brown, C.R.; Neate, S.L.; Taylor, K.L.; Weiland, T.J. Associations of Lifestyle, Medication, and Socio-Demographic Factors with Disability in People with Multiple Sclerosis: An International Cross-Sectional Study. PLoS ONE 2016, 11, e0161701. [Google Scholar] [CrossRef] [PubMed]
- Bjørnevik, K.; Chitnis, T.; Ascherio, A.; Munger, K.L. Polyunsaturated fatty acids and the risk of multiple sclerosis. Mult. Scler. J. 2017, 23, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Azary, S.; Schreiner, T.; Graves, J.; Waldman, A.; Belman, A.; Guttman, B.W.; Aaen, G.; Tillema, J.-M.; Mar, S.; Hart, J.; et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Bäärnhielm, M.; Olsson, T.; Alfredsson, L. Fatty fish intake is associated with decreased occurrence of multiple sclerosis. Mult. Scler. J. 2014, 20, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Tyry, T.; Salter, A.; Cofield, S.S.; Cutter, G.; Fox, R.; Marrie, R.A. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018, 90, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Shore, V.G.; Smith, M.E.; Perret, V.; Laskaris, M.A. Alterations in plasma lipoproteins and apolipoproteins in experimental allergic encephalomyelitis. J. Lipid Res. 1987, 28, 119–129. [Google Scholar] [PubMed]
- Newcombe, J.; Li, H.; Cuzner, M.L. Low density lipoprotein uptake by macrophages in multiple sclerosis plaques: Implications for pathogenesis. Neuropathol. Appl. Neurobiol. 1994, 20, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Hoftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative damage in multiple sclerosis lesions. Brain 2011, 134, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Boven, L.A.; Van Meurs, M.; Van Zwam, M.; Wierenga-Wolf, A.; Hintzen, R.Q.; Boot, R.G.; Aerts, J.M.; Amor, S.; Nieuwenhuis, E.E.; Laman, J.D. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 2006, 129, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Yepes, M.; Sandkvist, M.; Moore, E.G.; Bugge, T.H.; Strickland, D.K.; Lawrence, D.A. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor–related protein. J. Clin. Investig. 2003, 112, 1533–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaultier, A.; Wu, X.; Le Moan, N.; Takimoto, S.; Mukandala, G.; Akassoglou, K.; Campana, W.M.; Gonias, S.L. Low-density lipoprotein receptor-related protein 1 is an essential receptor for myelin phagocytosis. J. Cell Sci. 2009, 122, 1155–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podbielska, M.; Hogan, E. Molecular and immunogenic features of myelin lipids: Incitants or modulators of multiple sclerosis? Mult. Scler. J. 2009, 15, 1011–1029. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, R.; Nijland, P.G.; Dekker, N.; Witte, M.E.; Lopes-Pinheiro, M.A.; van het Hof, B.; Kooij, G.; Reijerkerk, A.; Dijkstra, C.; van van der Valk, P.; et al. Fingolimod attenuates ceramide-induced blood–brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes. Acta Neuropathol. 2012, 124, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Checa, A.; Khademi, M.; Sar, D.G.; Haeggström, J.Z.; Lundberg, J.O.; Piehl, F.; Olsson, T.; Wheelock, C.E. Hexosylceramides as intrathecal markers of worsening disability in multiple sclerosis. Mult. Scler. 2015, 21, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Obermeier, B.; Cotleur, A.; Love, A.; Takeshita, Y.; Sano, Y.; Kanda, T.; Ransohoff, R.M. Sphingosine 1 Phosphate at the Blood Brain Barrier: Can the Modulation of S1P Receptor 1 Influence the Response of Endothelial Cells and Astrocytes to Inflammatory Stimuli? PLoS ONE 2015, 10, e0133392. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Malchinkhuu, E.; Horiuchi, Y.; Mogi, C.; Tomura, H.; Tosaka, M.; Yoshimoto, Y.; Kuwabara, A.; Okajima, F. HDL-like lipoproteins in cerebrospinal fluid affect neural cell activity through lipoprotein-associated sphingosine 1-phosphate. Biochem. Biophys. Res. Commun. 2007, 359, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Kułakowska, A.; Żendzian-Piotrowska, M.; Baranowski, M.; Konończuk, T.; Drozdowski, W.; Górski, J.; Bucki, R. Intrathecal increase of sphingosine 1-phosphate at early stage multiple sclerosis. Neurosci. Lett. 2010, 477, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.M.; Sádaba, M.C.; Roldán, E.; Masjuan, J.; González-Porqué, P.; Villarrubia, N.; Espiño, M.; García-Trujillo, J.A.; Bootello, A.; Alvarez-Cermeño, J.C. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J. Clin. Investig. 2005, 115, 187–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, P.P.; Kanter, J.L.; Johnson, A.M.; Srinagesh, H.K.; Chang, E.-J.; Purdy, T.M.; van Haren, K.; Wikoff, W.R.; Kind, T.; et al. Identification of naturally occurring fatty acids of the myelin sheath that resolve neuroinflammation. Sci. Transl. Med. 2012, 4, 137ra73. [Google Scholar] [CrossRef] [PubMed]
- Mycko, M.P.; Sliwinska, B.; Cichalewska, M.; Cwiklinska, H.; Raine, C.S.; Selmaj, K.W. Brain glycolipids suppress T helper cells and inhibit autoimmune demyelination. J. Neurosci. 2014, 34, 8646–8658. [Google Scholar] [CrossRef] [PubMed]
- Saher, G.; Stumpf, S.K. Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim. Biophys. Acta 2015, 1851, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Itoh, Y.; Tassoni, A.; Ren, E.; Kaito, M.; Ohno, A.; Ao, Y.; Farkhondeh, V.; Johnsonbaugh, H.; Burda, J.; et al. Cell-specific and region-specific transcriptomics in the multiple sclerosis model: Focus on astrocytes. Proc. Natl. Acad. Sci. USA 2017, 115, E302–E309. [Google Scholar] [CrossRef] [PubMed]
- Lavrnja, I.; Smiljanic, K.; Savic, D.; Mladenovic-Djordjevic, A.; Tesovic, K.; Kanazir, S.; Pekovic, S. Expression profiles of cholesterol metabolism-related genes are altered during development of experimental autoimmune encephalomyelitis in the rat spinal cord. Sci. Rep. 2017, 7, 2702. [Google Scholar] [CrossRef] [PubMed]
- Baitsch, D.; Bock, H.H.; Engel, T.; Telgmann, R.; Muller-Tidow, C.; Varga, G.; Bot, M.; Herz, J.; Robenek, H.; von Eckardstein, A.; et al. Apolipoprotein E Induces Antiinflammatory Phenotype in Macrophages. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1160–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mailleux, J.; Vanmierlo, T.; Bogie, J.F.; Wouters, E.; Lütjohann, D.; Hendriks, J.J.; van Horssen, J. Active liver X receptor signaling in phagocytes in multiple sclerosis lesions. Mult. Scler. J. 2018, 24, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Howie, D.; Bokum, A. Ten; Necula, A.S.; Cobbold, S.P.; Waldmann, H. The role of lipid metabolism in T lymphocyte differentiation and survival. Front. Immunol. 2018, 8, 1949. [Google Scholar] [CrossRef] [PubMed]
- Mailleux, J.; Timmermans, S.; Nelissen, K.; Vanmol, J.; Vanmierlo, T.; van Horssen, J.; Bogie, J.F.J.; Hendriks, J.J.A. Low-Density Lipoprotein Receptor Deficiency Attenuates Neuroinflammation through the Induction of Apolipoprotein E. Front. Immunol. 2017, 8, 1701. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, S.; van Eden, C.G.; Schuurman, K.; van Strien, M.E.; Swaab, D.F.; Huitinga, I. Gender Differences in Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2014, 73, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, D.; Bandaru, V.V.R.; Calabresi, P.A.; Nath, A.; Haughey, N.J. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain 2008, 131, 3092–3102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidaurre, O.G.; Haines, J.D.; Katz Sand, I.; Adula, K.P.; Huynh, J.L.; McGraw, C.A.; Zhang, F.; Varghese, M.; Sotirchos, E.; Bhargava, P.; et al. Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain 2014, 137, 2271–2286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friese, M.A.; Schattling, B.; Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 2014, 10, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef]
- Rajda, C.; Pukoli, D.; Bende, Z.; Majláth, Z.; Vécsei, L. Excitotoxins, Mitochondrial and Redox Disturbances in Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 353. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, N.; Matthews, P.M.; Antel, J.P.; Preul, M.; Francis, G.; Arnold, D.L. Chemical pathology of acute demyelinating lesions and its correlation with disability. Ann. Neurol. 1995, 38, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Paling, D.; Golay, X.; Wheeler-Kingshott, C.; Kapoor, R.; Miller, D. Energy failure in multiple sclerosis and its investigation using MR techniques. J. Neurol. 2011, 258, 2113–2127. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.A.; Maghzal, G.J.; Khademi, M.; Piehl, F.; Ratzer, R.; Romme Christensen, J.; Sellebjerg, F.T.; Olsson, T.; Stocker, R. Absence of systemic oxidative stress and increased CSF prostaglandin F 2α in progressive MS. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e256. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, J.; Drexhage, J.A.R.; Flor, T.; Gerritsen, W.; van der Valk, P.; de Vries, H.E. Nrf2 and DJ1 are consistently upregulated in inflammatory multiple sclerosis lesions. Free Radic. Biol. Med. 2010, 49, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Morales Pantoja, I.E.; Hu, C.; Perrone-Bizzozero, N.I.; Zheng, J.; Bizzozero, O.A. Nrf2-dysregulation correlates with reduced synthesis and low glutathione levels in experimental autoimmune encephalomyelitis. J. Neurochem. 2016, 139, 640–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, I.-Y.; Lee, P.; Adany, P.; Hughes, A.J.; Belliston, S.; Denney, D.R.; Lynch, S.G. In vivo evidence of oxidative stress in brains of patients with progressive multiple sclerosis. Mult. Scler. J. 2017, 135245851771156. [Google Scholar] [CrossRef] [PubMed]
- Habas, A.; Hahn, J.; Wang, X.; Margeta, M. Neuronal activity regulates astrocytic Nrf2 signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 18291–18296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Kraats, C.; Killestein, J.; Popescu, V.; Rijkers, E.; Vrenken, H.; Lütjohann, D.; Barkhof, F.; Polman, C.H.; Teunissen, C.E. Oxysterols and cholesterol precursors correlate to magnetic resonance imaging measures of neurodegeneration in multiple sclerosis. Mult. Scler. 2014, 20, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Friese, M.A. Widespread synaptic loss in multiple sclerosis. Brain 2016, 139, 2–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jürgens, T.; Jafari, M.; Kreutzfeldt, M.; Bahn, E.; Brück, W.; Kerschensteiner, M.; Merkler, D. Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain 2016, 139, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, I.; Naessens, D.M.P.; Hametner, S.; Guldenaar, W.; Kooi, E.-J.; Geurts, J.J.G.; Baas, F.; Lassmann, H.; Ramaglia, V. Complement C3 on microglial clusters in multiple sclerosis occur in chronic but not acute disease: Implication for disease pathogenesis. Glia 2017, 65, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayo, L.; Trauger, S.A.; Blain, M.; Nadeau, M.; Patel, B.; Alvarez, J.I.; Mascanfroni, I.D.; Yeste, A.; Kivisäkk, P.; Kallas, K.; et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat. Med. 2014, 20, 1147–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothhammer, V.; Kenison, J.E.; Tjon, E.; Takenaka, M.C.; de Lima, K.A.; Borucki, D.M.; Chao, C.-C.; Wilz, A.; Blain, M.; Healy, L.; et al. Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, 2012–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giubilei, F.; Antonini, G.; Di Legge, S.; Sormani, M.P.; Pantano, P.; Antonini, R.; Sepe-Monti, M.; Caramia, F.; Pozzilli, C. Blood cholesterol and MRI activity in first clinical episode suggestive of multiple sclerosis. Acta Neurol. Scand. 2002, 106, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Zhornitsky, S.; McKay, K.A.; Metz, L.M.; Teunissen, C.E.; Rangachari, M. Cholesterol and markers of cholesterol turnover in multiple sclerosis: Relationship with disease outcomes. Mult. Scler. Relat. Disord. 2016, 5, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Jorissen, W.; Wouters, E.; Bogie, J.F.; Vanmierlo, T.; Noben, J.-P.; Sviridov, D.; Hellings, N.; Somers, V.; Valcke, R.; Vanwijmeersch, B.; et al. Relapsing-remitting multiple sclerosis patients display an altered lipoprotein profile with dysfunctional HDL. Sci. Rep. 2017, 7, 43410. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Fellows, K.; Browne, R.W.; Khare, P.; Krishnan Radhakrishnan, S.; Hagemeier, J.; Weinstock-Guttman, B.; Zivadinov, R.; Ramanathan, M. Interdependence of oxysterols with cholesterol profiles in multiple sclerosis. Mult. Scler. 2017, 23, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, B.; Laman, J.D. The in and out of monocytes in atherosclerotic plaques: Balancing inflammation through migration. Proc. Natl. Acad. Sci. USA 2004, 101, 11529–11530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrie, R.A.; Rudick, R.; Horwitz, R.; Cutter, G.; Tyry, T.; Campagnolo, D.; Vollmer, T. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 2010, 74, 1041–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalec, K.; McKay, K.A.; Patten, S.B.; Fisk, J.D.; Evans, C.; Tremlett, H.; Marrie, R.A. CIHR Team in Epidemiology and Impact of Comorbidity on Multiple Sclerosis (ECoMS) Comorbidity increases the risk of relapse in multiple sclerosis. Neurology 2017, 89, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Palavra, F.; Reis, F.; Marado, D.; Sena, A. (Eds.) Biomarkers of Cardiometabolic Risk, Inflammation and Disease; Springer International Publishing: Cham, Switzerland, 2015; ISBN 9783319160184. [Google Scholar]
- Sena, A.; Pedrosa, R.; Graça Morais, M. Therapeutic potential of lovastatin in multiple sclerosis. J. Neurol. 2003, 250, 754–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmer, T.; Key, L.; Durkalski, V.; Tyor, W.; Corboy, J.; Markovic-Plese, S.; Preiningerova, J.; Rizzo, M.; Singh, I. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet 2004, 363, 1607–1608. [Google Scholar] [CrossRef]
- Chataway, J.; Schuerer, N.; Alsanousi, A.; Chan, D.; MacManus, D.; Hunter, K.; Anderson, V.; Bangham, C.R.M.; Clegg, S.; Nielsen, C.; et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): A randomised, placebo-controlled, phase 2 trial. Lancet 2014, 383, 2213–2221. [Google Scholar] [CrossRef]
- Chan, D.; Binks, S.; Nicholas, J.M.; Frost, C.; Cardoso, M.J.; Ourselin, S.; Wilkie, D.; Nicholas, R.; Chataway, J. Effect of high-dose simvastatin on cognitive, neuropsychiatric, and health-related quality-of-life measures in secondary progressive multiple sclerosis: Secondary analyses from the MS-STAT randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 591–600. [Google Scholar] [CrossRef]
- Sena, A.; Pedrosa, R.; Ferret-Sena, V.; Almeida, R.; Andrade, M.L.; Morais, M.G.; Couderc, R. Interferon β1a therapy changes lipoprotein metabolism in patients with multiple sclerosis. Clin. Chem. Lab. Med. 2000, 38, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Uher, T.; Fellows, K.; Horakova, D.; Zivadinov, R.; Vaneckova, M.; Sobisek, L.; Tyblova, M.; Seidl, Z.; Krasensky, J.; Bergsland, N.; et al. Serum lipid profile changes predict neurodegeneration in interferon-β1a-treated multiple sclerosis patients. J. Lipid Res. 2017, 58, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Ottenlinger, F.M.; Mayer, C.A.; Ferreirós, N.; Schreiber, Y.; Schwiebs, A.; Schmidt, K.G.; Ackermann, H.; Pfeilschifter, J.M.; Radeke, H.H. Interferon-β Increases Plasma Ceramides of Specific Chain Length in Multiple Sclerosis Patients, Unlike Fingolimod or Natalizumab. Front. Pharmacol. 2016, 7, 412. [Google Scholar] [CrossRef] [PubMed]
- Del Boccio, P.; Pieragostino, D.; Di Ioia, M.; Petrucci, F.; Lugaresi, A.; De Luca, G.; Gambi, D.; Onofrj, M.; Di Ilio, C.; Sacchetta, P.; et al. Lipidomic investigations for the characterization of circulating serum lipids in multiple sclerosis. J. Proteom. 2011, 74, 2826–2836. [Google Scholar] [CrossRef] [PubMed]
- Villoslada, P.; Alonso, C.; Agirrezabal, I.; Kotelnikova, E.; Zubizarreta, I.; Pulido-Valdeolivas, I.; Saiz, A.; Comabella, M.; Montalban, X.; Villar, L.; et al. Metabolomic signatures associated with disease severity in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e321. [Google Scholar] [CrossRef] [PubMed]
- Lötsch, J.; Thrun, M.; Lerch, F.; Brunkhorst, R.; Schiffmann, S.; Thomas, D.; Tegder, I.; Geisslinger, G.; Ultsch, A. Machine-Learned Data Structures of Lipid Marker Serum Concentrations in Multiple Sclerosis Patients Differ from Those in Healthy Subjects. Int. J. Mol. Sci. 2017, 18, 1217. [Google Scholar] [CrossRef] [PubMed]
- Quintana, F.J.; Farez, M.F.; Viglietta, V.; Iglesias, A.H.; Merbl, Y.; Izquierdo, G.; Lucas, M.; Basso, A.S.; Khoury, S.J.; Lucchinetti, C.F.; et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2008, 105, 18889–18894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakshi, R.; Yeste, A.; Patel, B.; Tauhid, S.; Tummala, S.; Rahbari, R.; Chu, R.; Regev, K.; Kivisäkk, P.; Weiner, H.L.; et al. Serum lipid antibodies are associated with cerebral tissue damage in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e200. [Google Scholar] [CrossRef] [PubMed]
- Sena, A.; Bendtzen, K.; Cascais, M.J.; Pedrosa, R.; Ferret-Sena, V.; Campos, E. Influence of apolipoprotein E plasma levels and tobacco smoking on the induction of neutralising antibodies to interferon-β. J. Neurol. 2010, 257, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-Q.; Sempowski, G.D.; McKenna, S.E.; Laskowitz, D.T.; Colton, C.A.; Vitek, M.P. Apolipoprotein E-derived peptides ameliorate clinical disability and inflammatory infiltrates into the spinal cord in a murine model of multiple sclerosis. J. Pharmacol. Exp. Ther. 2006, 318, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Michalek, R.D.; Gerriets, V.A.; Jacobs, S.R.; Macintyre, A.N.; MacIver, N.J.; Mason, E.F.; Sullivan, S.A.; Nichols, A.G.; Rathmell, J.C. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. J. Immunol. 2011, 186, 3299–3303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, E.L.; Poffenberger, M.C.; Chang, C.-H.; Jones, R.G. Fueling Immunity: Insights into Metabolism and Lymphocyte Function. Science 2013, 342, 1242454. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; De Rosa, V.; Carrieri, P.B.; Montella, S.; Bruzzese, D.; Porcellini, A.; Procaccini, C.; La Cava, A.; Matarese, G. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat. Med. 2014, 20, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Gerriets, V.A.; Kishton, R.J.; Johnson, M.O.; Cohen, S.; Siska, P.J.; Nichols, A.G.; Warmoes, M.O.; de Cubas, A.A.; MacIver, N.J.; Locasale, J.W.; et al. Foxp3 and Toll-like receptor signaling balance Tregcell anabolic metabolism for suppression. Nat. Immunol. 2016, 17, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, C.; Carbone, F.; De Rosa, V.; Colamatteo, A.; Galgani, M.; Perna, F.; Lanzillo, R.; Brescia Morra, V.; Orefice, G.; et al. Immunometabolic profiling of T cells from patients with relapsing-remitting multiple sclerosis reveals an impairment in glycolysis and mitochondrial respiration. Metabolism 2017, 77, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, Y.; Fang, J.; Chen, Y.; Li, H.; Gao, K. Dimethyl fumarate inhibits the expression and function of hypoxia-inducible factor-1α (HIF-1α). Biochem. Biophys. Res. Commun. 2014, 448, 303–307. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, S.; Haghikia, A.; Gold, R.; Linker, R.A. Dietary fatty acids and susceptibility to multiple sclerosis. Mult. Scler. J. 2018, 24, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.-C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothhammer, V.; Borucki, D.M.; Garcia Sanchez, M.I.; Mazzola, M.A.; Hemond, C.C.; Regev, K.; Paul, A.; Kivisäkk, P.; Bakshi, R.; Izquierdo, G.; et al. Dynamic regulation of serum aryl hydrocarbon receptor agonists in MS. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.K.; Bilgin, A.; Lovejoy, D.B.; Tan, V.; Bustamante, S.; Taylor, B.V.; Bessede, A.; Brew, B.J.; Guillemin, G.J. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci. Rep. 2017, 7, 41473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Procaccini, C.; Santopaolo, M.; Faicchia, D.; Colamatteo, A.; Formisano, L.; De Candia, P.; Galgani, M.; De Rosa, V.; Matarese, G. Role of metabolism in neurodegenerative disorders. Metabolism 2016, 65, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.J.; Sie, C.; Schumann, E.; Witte, A.-K.; Dressel, R.; van den Brandt, J.; Reichardt, H.M. The Insulin Receptor Plays a Critical Role in T Cell Function and Adaptive Immunity. J. Immunol. 2017, 198, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Negrotto, L.; Farez, M.F.; Correale, J. Immunologic Effects of Metformin and Pioglitazone Treatment on Metabolic Syndrome and Multiple Sclerosis. JAMA Neurol. 2016, 73, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sena, A.; Sarliève, L.L.; Rebel, G. Brain myelin of genetically obese mice. J. Neurol. Sci. 1985, 68, 233–243. [Google Scholar] [CrossRef]
- Fleck, A.K.; Schuppan, D.; Wiendl, H.; Klotz, L. Gut–CNS-axis as possibility to modulate inflammatory disease activity—Implications for multiple sclerosis. Int. J. Mol. Sci. 2017, 18, 1526. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Sowa, M.; Medrek, A.; Madej, P.; Michlicka, W.; Dobrakowski, P. Does the Gut Microbiota Influence Immunity and Inflammation in Multiple Sclerosis Pathophysiology? J. Immunol. Res. 2017, 2017, 7904821. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogen, W.J.; Laman, J.D.; ’t Hart, B.A. Modulation of Multiple Sclerosis and Its Animal Model Experimental Autoimmune Encephalomyelitis by Food and Gut Microbiota. Front. Immunol. 2017, 8, 1081. [Google Scholar] [CrossRef] [PubMed]
- Trott, S.; King, I.L. An introduction to the microbiome and MS. Mult. Scler. J. 2018, 24, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Berer, K.; Mues, M.; Koutrolos, M.; AlRasbi, Z.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasitschka, F.; Giese, T.; Paparella, M.; Kurzhals, S.R.; Wabnitz, G.; Jacob, K.; Gras, J.; Bode, K.A.; Heninger, A.-K.; Sziskzai, T.; et al. Human monocytes downregulate innate response receptors following exposure to the microbial metabolite n-butyrate. Immunity Inflamm. Dis. 2017, 5, 480–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.-H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2016, 44, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Noto, D.; Kaga, N.; Chiba, A.; Miyake, S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS ONE 2017, 12, e0173032. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Soldan, M.M.P.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremlett, H.; Fadrosh, D.W.; Faruqi, A.A.; Zhu, F.; Hart, J.; Roalstad, S.; Graves, J.; Lynch, S.; Waubant, E. US Network of Pediatric MS Centers Gut microbiota in early pediatric multiple sclerosis: A case-control study. Eur. J. Neurol. 2016, 23, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E.; et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosorich, I.; Dalla-Costa, G.; Sorini, C.; Ferrarese, R.; Messina, M.J.; Dolpady, J.; Radice, E.; Mariani, A.; Testoni, P.A.; Canducci, F.; et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci. Adv. 2017, 3, e1700492. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Toth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 2016, 6, e774. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Prinz, M. Communicating systems in the body: How microbiota and microglia cooperate. Immunology 2017, 150, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Gille, C.; Göktas, Ö.; Reißhauer, A.; Neuhaus, J.; Weylandt, K.-H.; Guschin, A.; Bock, M. Reduced Mass and Diversity of the Colonic Microbiome in Patients with Multiple Sclerosis and Their Improvement with Ketogenic Diet. Front. Microbiol. 2017, 8, 1141. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Mendozzi, L.; Rossi, V.; Mazzali, F.; Piancone, F.; LaRosa, F.; Marventano, I.; Caputo, D.; Felis, G.E.; Clerici, M. Immunological and Clinical Effect of Diet Modulation of the Gut Microbiome in Multiple Sclerosis Patients: A Pilot Study. Front. Immunol. 2017, 8, 1391. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Faraco, G.; Brea, D.; Garcia-Bonilla, L.; Wang, G.; Racchumi, G.; Chang, H.; Buendia, I.; Santisteban, M.M.; Segarra, S.G.; et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat. Neurosci. 2018, 21, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Barbier, O.; Torra, I.P.; Duguay, Y.; Blanquart, C.; Fruchart, J.-C.; Glineur, C.; Staels, B. Pleiotropic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, E.; Chinetti-Gbaguidi, G.; Staels, B. Regulation of macrophage functions by PPAR-α, PPAR-γ, and LXRs in mice and men. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Daynes, R.A.; Jones, D.C. Emerging roles of PPARS in inflammation and immunity. Nat. Rev. Immunol. 2002, 2, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Ricote, M.; Valledor, A.F.; Glass, C.K. Decoding Transcriptional Programs Regulated by PPARs and LXRs in the Macrophage: Effects on Lipid Homeostasis, Inflammation, and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Kidani, Y.; Bensinger, S.J. Liver X receptor and peroxisome proliferator-activated receptor as integrators of lipid homeostasis and immunity. Immunol. Rev. 2012, 249, 72–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racke, M.K.; Gocke, A.R.; Muir, M.; Diab, A.; Drew, P.D.; Lovett-Racke, A.E. Nuclear receptors and autoimmune disease: The potential of PPAR agonists to treat multiple sclerosis. J. Nutr. 2006, 136, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.E.; Ousman, S.S.; Sobel, R.A.; Zuniga, L.; Baranzini, S.E.; Youssef, S.; Crowell, A.; Loh, J.; Oksenberg, J.; Steinman, L. Peroxisome proliferator–activated receptor (PPAR)α expression in T cells mediates gender differences in development of T cell–mediated autoimmunity. J. Exp. Med. 2007, 204, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotz, L.; Burgdorf, S.; Dani, I.; Saijo, K.; Flossdorf, J.; Hucke, S.; Alferink, J.; Nowak, N.; Novak, N.; Beyer, M.; et al. The nuclear receptor PPAR γ selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J. Exp. Med. 2009, 206, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.E.; Bhat, R.; Straus, D.S.; Sobel, R.A.; Axtell, R.; Johnson, A.; Nguyen, K.; Mukundan, L.; Moshkova, M.; Dugas, J.C.; et al. Peroxisome proliferator–activated receptor δ limits the expansion of pathogenic Th cells during central nervous system autoimmunity. J. Exp. Med. 2010, 207, 1599–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unoda, K.; Doi, Y.; Nakajima, H.; Yamane, K.; Hosokawa, T.; Ishida, S.; Kimura, F.; Hanafusa, T. Eicosapentaenoic acid (EPA) induces peroxisome proliferator-activated receptors and ameliorates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2013, 256, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.J.; Kanakasabai, S.; Chearwae, W.; Chakraborty, S. PPAR Regulation of Inflammatory Signaling in CNS Diseases. PPAR Res. 2008, 2008, 658520. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, D.V.; Aleshin, S.E.; Astakhova, A.A.; Sergeeva, M.G.; Reiser, G. Regulation of peroxisome proliferator-activated receptors (PPAR) α and -γ of rat brain astrocytes in the course of activation by toll-like receptor agonists. J. Neurochem. 2015, 134, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hucke, S.; Floßdorf, J.; Grützke, B.; Dunay, I.R.; Frenzel, K.; Jungverdorben, J.; Linnartz, B.; Mack, M.; Peitz, M.; Brüstle, O.; et al. Licensing of myeloid cells promotes central nervous system autoimmunity and is controlled by peroxisome proliferator-activated receptor γ. Brain 2012, 135, 1586–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogie, J.F.; Jorissen, W.; Mailleux, J.; Nijland, P.G.; Zelcer, N.; Vanmierlo, T.; Van Horssen, J.; Stinissen, P.; Hellings, N.; Hendriks, J.J. Myelin alters the inflammatory phenotype of macrophages by activating PPARs. Acta Neuropathol. Commun. 2013, 1, 43. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Endo, T.; Kanda, T.; Nakamura, H.; Yamasaki, S.; Yamakawa, T. Synthesis of a novel human PPARδ selective agonist and its stimulatory effect on oligodendrocyte differentiation. Bioorg. Med. Chem. Lett. 2011, 21, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Jana, M.; Mondal, S.; Gonzalez, F.J.; Pahan, K. Gemfibrozil, a Lipid-lowering Drug, Increases Myelin Genes in Human Oligodendrocytes via Peroxisome Proliferator-activated Receptor-β. J. Biol. Chem. 2012, 287, 34134–34148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanakasabai, S.; Pestereva, E.; Chearwae, W.; Gupta, S.K.; Ansari, S.; Bright, J.J. PPARγ Agonists Promote Oligodendrocyte Differentiation of Neural Stem Cells by Modulating Stemness and Differentiation Genes. PLoS ONE 2012, 7, e50500. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.D.; Leisewitz, A.V.; Jung, J.E.; Cassina, P.; Barbeito, L.; Inestrosa, N.C.; Bronfman, M. PPARγ activators induce growth arrest and process extension in B12 oligodendrocyte-like cells and terminal differentiation of cultured oligodendrocytes. J. Neurosci. Res. 2003, 72, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, A.; Bianchi, D.; Magnaghi, V.; Minghetti, L. Peroxisome proliferator-activated receptor-gamma agonists promote differentiation and antioxidant defenses of oligodendrocyte progenitor cells. J. Neuropathol. Exp. Neurol. 2009, 68, 797–808. [Google Scholar] [CrossRef] [PubMed]
- De Nuccio, C.; Bernardo, A.; De Simone, R.; Mancuso, E.; Magnaghi, V.; Visentin, S.; Minghetti, L. Peroxisome Proliferator-Activated Receptor γ Agonists Accelerate Oligodendrocyte Maturation and Influence Mitochondrial Functions and Oscillatory Ca2+ Waves. J. Neuropathol. Exp. Neurol. 2011, 70, 900–912. [Google Scholar] [CrossRef] [PubMed]
- De Nuccio, C.; Bernardo, A.; Cruciani, C.; De Simone, R.; Visentin, S.; Minghetti, L. Peroxisome proliferator activated receptor-γ agonists protect oligodendrocyte progenitors against tumor necrosis factor-alpha-induced damage: Effects on mitochondrial functions and differentiation. Exp. Neurol. 2015, 271, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, A.; Giammarco, M.L.; De Nuccio, C.; Ajmone-Cat, M.A.; Visentin, S.; De Simone, R.; Minghetti, L. Docosahexaenoic acid promotes oligodendrocyte differentiation via PPAR-γ signalling and prevents tumor necrosis factor-α-dependent maturational arrest. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Vallée, J.-N.; Guillevin, R.; Lecarpentier, Y. Interactions Between the Canonical WNT/Beta-Catenin Pathway and PPAR γ on Neuroinflammation, Demyelination, and Remyelination in Multiple Sclerosis. Cell Mol. Neurobiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Farioli-Vecchioli, S.; Cerù, M.P. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience 2004, 123, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Warden, A.; Truitt, J.; Merriman, M.; Ponomareva, O.; Jameson, K.; Ferguson, L.B.; Mayfield, R.D.; Harris, R.A. Localization of PPAR isotypes in the adult mouse and human brain. Sci. Rep. 2016, 6, 27618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleshin, S.; Strokin, M.; Sergeeva, M.; Reiser, G. Peroxisome proliferator-activated receptor (PPAR)β/δ, a possible nexus of PPARα- and PPARγ-dependent molecular pathways in neurodegenerative diseases: Review and novel hypotheses. Neurochem. Int. 2013, 63, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, E.; Di Loreto, S.; D’Angelo, B.; Cristiano, L.; d’Angelo, M.; Antonosante, A.; Fidoamore, A.; Golini, R.; Cinque, B.; Cifone, M.G.; et al. The PPARβ/δ Agonist GW0742 Induces Early Neuronal Maturation of Cortical Post-Mitotic Neurons: Role of PPARβ/δ in Neuronal Maturation. J. Cell Physiol. 2016, 231, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Yadav, A.; Chaturvedi, R.K. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem. Biophys. Res. Commun. 2017, 483, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.L.; Wang, Z.; Aljabar, P.; Ball, G.; Mirza, G.; Saxena, A.; Counsell, S.J.; Hajnal, J.V.; Montana, G.; Edwards, A.D. Machine learning shows association between genetic variability inPPARGand cerebral connectivity in preterm infants. Proc. Natl. Acad. Sci. USA 2017, 114, 13744–13749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omeragic, A.; Hoque, M.T.; Choi, U.-Y.; Bendayan, R. Peroxisome proliferator-activated receptor-gamma: Potential molecular therapeutic target for HIV-1-associated brain inflammation. J. Neuroinflamm. 2017, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yao, H.; Chen, Y.; Sun, L.; Li, Y.; Ma, X.; Duan, S.; Li, X.; Xiang, R.; Han, J.; et al. Inhibition of Glutathione Production Induces Macrophage CD36 Expression and Enhances Cellular-oxidized Low Density Lipoprotein (oxLDL) Uptake. J. Biol. Chem. 2015, 290, 21788–21799. [Google Scholar] [CrossRef] [PubMed]
- Polvani, S.; Tarocchi, M.; Galli, A. PPARγ and Oxidative Stress: Con(β) Catenating NRF2 and FOXO. PPAR Res. 2012, 2012, 641087. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-J.; Lee, E.-J.; Park, J.-S.; Kim, S.-N.; Park, E.-M.; Kim, H.-S. Anti-inflammatory mechanism of galangin in lipopolysaccharide-stimulated microglia: Critical role of PPAR-γ signaling pathway. Biochem. Pharmacol. 2017, 144, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Sekulic-Jablanovic, M.; Petkovic, V.; Wright, M.B.; Kucharava, K.; Huerzeler, N.; Levano, S.; Brand, Y.; Leitmeyer, K.; Glutz, A.; Bausch, A.; et al. Effects of peroxisome proliferator activated receptors (PPAR)-γ and -α agonists on cochlear protection from oxidative stress. PLoS ONE 2017, 12, e0188596. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Xiao, Y.; Ma, S.; Liu, Q.; Dang, S.; Jin, M.; Shi, Y.; Wan, B.; Zhang, Y. Inhibition of 12/15-lipoxygenase by baicalein induces microglia PPARβ/δ: A potential therapeutic role for CNS autoimmune disease. Cell. Death Dis. 2013, 4, e569. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.; Helling, N.; Hilla, A.; Heskamp, A.; Issberner, A.; Hildebrandt, T.; Kohne, Z.; Küry, P.; Berndt, C.; Aktas, O.; et al. Early alpha-lipoic acid therapy protects from degeneration of the inner retinal layers and vision loss in an experimental autoimmune encephalomyelitis-optic neuritis model. J. Neuroinflamm. 2018, 15, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.-C.; Tsai, C.-P.; Lee, C.-L.; Chen, S.-Y.; Lin, G.-J.; Yen, M.-H.; Sytwu, H.-K.; Chen, S.-J. α-Lipoic acid enhances endogenous peroxisome-proliferator-activated receptor-γ to ameliorate experimental autoimmune encephalomyelitis in mice. Clin. Sci. 2013, 125, 329–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotz, L.; Schmidt, M.; Giese, T.; Sastre, M.; Knolle, P.; Klockgether, T.; Heneka, M.T. Proinflammatory stimulation and pioglitazone treatment regulate peroxisome proliferator-activated receptor gamma levels in peripheral blood mononuclear cells from healthy controls and multiple sclerosis patients. J. Immunol. 2005, 175, 4948–4955. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Schmidt, S.; Heun, R.; Klockgether, T.; Kölsch, H. Association of the PPARγ gene polymorphism Pro12Ala with delayed onset of multiple sclerosis. Neurosci. Lett. 2009, 449, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Pershadsingh, H.A.; Heneka, M.T.; Saini, R.; Amin, N.M.; Broeske, D.J.; Feinstein, D.L. Effect of pioglitazone treatment in a patient with secondary multiple sclerosis. J. Neuroinflamm. 2004, 1, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, C.C.; Shukla, D.K.; Stebbins, G.T.; Skias, D.D.; Jeffery, D.R.; Stefoski, D.; Katsamakis, G.; Feinstein, D.L. A pilot test of pioglitazone as an add-on in patients with relapsing remitting multiple sclerosis. J. Neuroimmunol. 2009, 211, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.K.; Kaiser, C.C.; Stebbins, G.T.; Feinstein, D.L. Effects of pioglitazone on diffusion tensor imaging indices in multiple sclerosis patients. Neurosci. Lett. 2010, 472, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Szalardy, L.; Zadori, D.; Tanczos, E.; Simu, M.; Bencsik, K.; Vecsei, L.; Klivenyi, P. Elevated levels of PPAR-γ in the cerebrospinal fluid of patients with multiple sclerosis. Neurosci. Lett. 2013, 554, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Rha, G.B.; Han, M.-J.; Eum, S.Y.; András, I.E.; Zhong, Y.; Hennig, B.; Toborek, M. PPARα and PPARgγ effectively protect against HIV-induced inflammatory responses in brain endothelial cells. J. Neurochem. 2008, 107, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Zhao, H.; Jiao, B.; Liu, F. EPA and DHA increased PPARγ expression and deceased integrin-linked kinase and integrin β1 expression in rat glomerular mesangial cells treated with lipopolysaccharide. Biosci. Trends 2014, 8, 120–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverstein, R.L.; Febbraio, M. CD36, a Scavenger Receptor Involved in Immunity, Metabolism, Angiogenesis, and Behavior. Sci. Signal. 2009, 2, re3. [Google Scholar] [CrossRef] [PubMed]
- Park, L.; Zhou, J.; Zhou, P.; Pistick, R.; El Jamal, S.; Younkin, L.; Pierce, J.; Arreguin, A.; Anrather, J.; Younkin, S.G.; et al. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc. Natl. Acad. Sci. USA 2013, 110, 3089–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferret-Sena, V.; Maia E Silva, A.; Sena, A.; Cavaleiro, I.; Vale, J.; Derudas, B.; Chinetti-Gbaguidi, G.; Staels, B. Natalizumab Treatment Modulates Peroxisome Proliferator-Activated Receptors Expression in Women with Multiple Sclerosis. PPAR Res. 2016, 2016, 5716415. [Google Scholar] [CrossRef] [PubMed]
- Sarov-Blat, L.; Kiss, R.S.; Haidar, B.; Kavaslar, N.; Jaye, M.; Bertiaux, M.; Steplewski, K.; Hurle, M.R.; Sprecher, D.; McPherson, R.; et al. Predominance of a Proinflammatory Phenotype in Monocyte-Derived Macrophages From Subjects With Low Plasma HDL-Cholesterol. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1115–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikary, T.; Wortmann, A.; Schumann, T.; Finkernagel, F.; Lieber, S.; Roth, K.; Toth, P.M.; Diederich, W.E.; Nist, A.; et al. The transcriptional PPARβ/δ network in human macrophages defines a unique agonist-induced activation state. Nucleic Acids Res. 2015, 43, 5033–5051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hla, T.; Brinkmann, V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology 2011, 76, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Ferret-Sena, V.; Capela, C.; Pedrosa, R.; Salgado, V.; Derudas, B.; Staels, B.; Sena, A. Fingolimod treatment increase peroxisome proliferator-activated receptor (PPAR) γ and CD36 receptor gene expression in blood leukocytes of multiple sclerosis patients. Mult. Scler. J. 2017, 23, 427–679. [Google Scholar]
- Zhong, Q.; Zhao, S.; Yu, B.; Wang, X.; Matyal, R.; Li, Y.; Jiang, Z. High-density Lipoprotein Increases the Uptake of Oxidized Low Density Lipoprotein via PPARγ/CD36 Pathway in Inflammatory Adipocytes. Int. J. Biol. Sci. 2015, 11, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Sini, S.; Deepa, D.; Harikrishnan, S.; Jayakumari, N. High-density lipoprotein from subjects with coronary artery disease promotes macrophage foam cell formation: Role of scavenger receptor CD36 and ERK/MAPK signaling. Mol. Cell Biochem. 2017, 427, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Sugai, H.; Kurosaki, H.; Ishikawa, M.; Funaki, A.; Kimura, Y.; Ueno, K. The effect of sex hormones on peroxisome proliferator-activated receptor gamma expression and activity in mature adipocytes. Biol. Pharm. Bull. 2013, 36, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, O.; Ballesteros, I.; Cuartero, M.I.; Moraga, A.; Pradillo, J.M.; Ramírez-Franco, J.; Bartolomé-Martín, D.; Pascual, D.; Torres, M.; Sánchez-Prieto, J.; et al. Daidzein has neuroprotective effects through ligand-binding-independent PPARγ activation. Neurochem. Int. 2012, 61, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, H.S.; Lee, J.U.; Bothwell, A.L.M.; Choi, J.M. Sex-based selectivity of PPARγ regulation in Th1, Th2, and Th17 differentiation. Int. J. Mol. Sci. 2016, 17, 1347. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Choi, J.-M. Sex-specific regulation of immune responses by PPARs. Exp. Mol. Med. 2017, 49, e364. [Google Scholar] [CrossRef] [PubMed]
- Sena, A.; Couderc, R.; Vasconcelos, J.C.; Ferret-Sena, V.; Pedrosa, R. Oral contraceptive use and clinical outcomes in patients with multiple sclerosis. J. Neurol. Sci. 2012, 317, 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bove, R.; Chitnis, T. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult. Scler. J. 2014, 20, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, M.; Guo, Y.; Song, Z.; Liu, B. 1,25-Dihydroxyvitamin D3 Promotes High Glucose-Induced M1 Macrophage Switching to M2 via the VDR-PPARγ Signaling Pathway. Biomed. Res. Int. 2015, 2015, 157834 1–157834 14. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Zhang, S.; Lu, W.; Wu, F.; Yin, X.; Yu, D.; Pan, Q.; Li, H. Regulation of Insulin Resistance and Adiponectin Signaling in Adipose Tissue by Liver X Receptor Activation Highlights a Cross-Talk with PPARγ. PLoS ONE 2014, 9, e101269. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Woo, M.-S.; Qin, L.; Ma, T.; Beltran, C.D.; Bao, Y.; Bailey, J.A.; Corbett, D.; Ratan, R.R.; Lahiri, D.K.; et al. Daidzein Augments Cholesterol Homeostasis via ApoE to Promote Functional Recovery in Chronic Stroke. J. Neurosci. 2015, 35, 15113–15126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranhotra, H.S. Gut Microbiota and Host Nuclear Receptors Signalling. Nucl. Recept. Res. 2017, 4, 10136. [Google Scholar] [CrossRef] [PubMed]
- Ohue-Kitano, R.; Yasuoka, Y.; Goto, T.; Kitamura, N.; Park, S.-B.; Kishino, S.; Kimura, I.; Kasubuchi, M.; Takahashi, H.; Li, Y.; et al. α-Linolenic acid-derived metabolites from gut lactic acid bacteria induce differentiation of anti-inflammatory M2 macrophages through G protein-coupled receptor 40. FASEB J. 2018, 32, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Byndloss, M.X.; Olsan, E.E.; Rivera-Chávez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017, 357, 570–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomas, J.; Mulet, C.; Saffarian, A.; Cavin, J.-B.; Ducroc, R.; Regnault, B.; Kun Tan, C.; Duszka, K.; Burcelin, R.; Wahli, W.; et al. High-fat diet modifies the PPAR-γ pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc. Natl. Acad. Sci. USA 2016, 113, E5934–E5943. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, I.; Suryawanshi, A.; Hong, Y.; Ranganathan, P.; Shanmugam, A.; Ahmad, S.; Swafford, D.; Manicassamy, B.; Ramesh, G.; Koni, P.A.; et al. Homeostatic PPARα Signaling Limits Inflammatory Responses to Commensal Microbiota in the Intestine. J. Immunol. 2016, 196, 4739–4749. [Google Scholar] [CrossRef] [PubMed]
- Corthals, A.P. Multiple sclerosis is not a disease of the immune system. Q. Rev. Biol. 2011, 86, 287–321. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferret-Sena, V.; Capela, C.; Sena, A. Metabolic Dysfunction and Peroxisome Proliferator-Activated Receptors (PPAR) in Multiple Sclerosis. Int. J. Mol. Sci. 2018, 19, 1639. https://doi.org/10.3390/ijms19061639
Ferret-Sena V, Capela C, Sena A. Metabolic Dysfunction and Peroxisome Proliferator-Activated Receptors (PPAR) in Multiple Sclerosis. International Journal of Molecular Sciences. 2018; 19(6):1639. https://doi.org/10.3390/ijms19061639
Chicago/Turabian StyleFerret-Sena, Véronique, Carlos Capela, and Armando Sena. 2018. "Metabolic Dysfunction and Peroxisome Proliferator-Activated Receptors (PPAR) in Multiple Sclerosis" International Journal of Molecular Sciences 19, no. 6: 1639. https://doi.org/10.3390/ijms19061639
APA StyleFerret-Sena, V., Capela, C., & Sena, A. (2018). Metabolic Dysfunction and Peroxisome Proliferator-Activated Receptors (PPAR) in Multiple Sclerosis. International Journal of Molecular Sciences, 19(6), 1639. https://doi.org/10.3390/ijms19061639