Influence of Bacterial Biofilm Polysaccharide Structure on Interactions with Antimicrobial Peptides: A Study on Klebsiella pneumoniae
Abstract
:1. Introduction
2. Results and Discussion
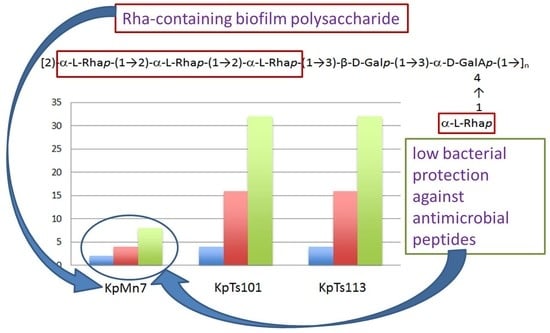
2.1. Interaction of Biofilms EPOLs and BMAP-27 AMP
2.2. Interaction of Biofilms EPOLs and Bac7(1–35) AMP
3. Materials and Methods
3.1. Bacterial Strains and Biofilm Culture
3.2. Antimicrobial Susceptibility Testing
3.3. Circular Dichroism Spectroscopy
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPs | Antimicrobial Peptides |
| MH | Müller Hinton solid medium |
| EPOLs | Extracellular Polysaccharides |
| MIC | Minimum Inhibitory Concentration |
| CD | Circular Dichroism |
References
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Branda, S.S.; Vik, Å.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Branda, S.S.; Gonzàlez-Pastor, J.E.; Ben-Yehuda, S.; Losick, R.; Kolter, R. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2001, 98, 11621–11626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mah, T.-F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C. EPS—Then and Now. Microorganisms 2016, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Lembre, P.; Lorentz, C.; Di Martino, P. Exopolysaccharides of the Biofilm Matrix: A Complex Biophysical World. In The Complex World of Polysaccharides; Karunaratne, D.N., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef] [Green Version]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The Roles of Antimicrobial Peptides in Innate Host Defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef] [PubMed]
- Foschiatti, M.; Cescutti, P.; Tossi, A.; Rizzo, R. Inhibition of cathelicidin activity by bacterial exopolysaccharides. Mol. Microbiol. 2009, 72, 1137–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herasimenka, Y.; Benincasa, M.; Mattiuzzo, M.; Cescutti, P.; Gennaro, R.; Rizzo, R. Interaction of antimicrobial peptides with bacterial polysaccharides from lung pathogens. Peptides 2005, 26, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.; Mattiuzzo, M.; Herasimenka, Y.; Cescutti, P.; Rizzo, R.; Gennaro, R. Activity of antimicrobial peptides in the presence of polysaccharides produced by pulmonary pathogens. J. Pept. Sci. 2009, 15, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.; Lagatolla, C.; Dolzani, L.; Milan, A.; Pacor, S.; Liut, G.; Tossi, A.; Cescutti, P.; Rizzo, R. Biofilms from Klebsiella pneumoniae: Matrix Polysaccharide Structure and Interactions with Antimicrobial Peptides. Microorganisms 2016, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cescutti, P.; De Benedetto, G.; Rizzo, R. Structural determination of the polysaccharide isolated from biofilms produced by a clinical strain of Klebsiella pneumoniae. Carbohydr. Res. 2016, 430, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kubler-Kielb, J.; Vinogradov, E.; Ng, W.-I.; Maczynska, B.; Junk, A.; Bartoszewicz, M.; Zelazny, A.; Bennett, J.; Schneerson, R. The capsular polysaccharide and lipopolysaccharide structures of two carbapenem resistant Klebsiella pneumoniae outbreak isolates. Carbohydr. Res. 2013, 369, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Mardirossian, M.; Grzela, R.; Giglione, C.; Meinnel, T.; Gennaro, R.; Mergaert, P.; Scocchi, M. The host antimicrobial peptide Bac7(1–35) binds to bacterial ribosomal proteins and inhibits protein synthesis. Cell Chem. Biol. 2014, 21, 1639–1647. [Google Scholar] [CrossRef]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, alpha-helical antimicrobial peptides Biopolymers. Pept. Sci. 2000, 55, 4–30. [Google Scholar] [CrossRef]
- Sonnichsen, F.D.; Van Eyk, J.E.; Hodges, R.S.; Sykes, B.D. Effect of trifluoroethanol on protein secondary structure: An NMR and CD study using a synthetic actin peptide. Biochemistry 1992, 31, 8790–8798. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.L.S.; Miles, A.J.; Whitmore, L.; Wallace, B.A. Distinct circular dichroism spectroscopic signatures of polyproline II and unordered secondary structures: Applications in secondary structure analyses. Protein Sci. 2014, 23, 1765–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and Analyzing Static Biofilms. Curr. Protoc. Microbiol. 2005. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; CLSI Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Juban, M.M.; Javadpour, M.M.; Barkley, M.D. Circular Dichroism Studies of Secondary Structure of Peptides. In Antibacterial Peptide Protocols. Methods in Molecular Biology™; Shafer, W.M., Ed.; Humana Press: Totowa, NJ, USA, 1997; Volume 78, pp. 73–78. [Google Scholar]
- Chen, Y.-H.; Yang, J.T.; Chau, K.H. Determination of the helix and β Form of Proteins in Aqueous Solution by Circular Dichroism. Biochemistry 1974, 13, 3350–3359. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, A.J.; Davies, D.B. Type-specific carbohydrate antigens of pathogenic bacteria. Part 1: Enterobacteriaceae. Carbohydr. Polym. 1991, 14, 241–279. [Google Scholar] [CrossRef]









| K. pneumoniae Strain | Biofilm Polysaccharide Structure | Reference |
|---|---|---|
| KpTs101 |  | [14] |
| KpTs113 |  | [15] |
| KpMn7 |  | [16] |
| Antimicrobial Peptide | Aminoacid Sequence | |
| BMAP-27 | GRFKRFRKKFKKLFKKLSPVIPLLHL-NH2 | |
| Bac7(1-35) | RRIRPRPPRLPRPRPRPLPFPRPGPRPIPRPLPFP-NH2 |
| Bacterial Strain | BMAP-27 | Bac7(1–35) |
|---|---|---|
| KpMn7 | 4 µM | 4 µM |
| KpTs101 | 4 µM | 4 µM |
| KpTs113 | 4 µM | 4 µM |
| E. coli ML-35 | 2 µM | 2 µM |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellich, B.; Lagatolla, C.; Tossi, A.; Benincasa, M.; Cescutti, P.; Rizzo, R. Influence of Bacterial Biofilm Polysaccharide Structure on Interactions with Antimicrobial Peptides: A Study on Klebsiella pneumoniae. Int. J. Mol. Sci. 2018, 19, 1685. https://doi.org/10.3390/ijms19061685
Bellich B, Lagatolla C, Tossi A, Benincasa M, Cescutti P, Rizzo R. Influence of Bacterial Biofilm Polysaccharide Structure on Interactions with Antimicrobial Peptides: A Study on Klebsiella pneumoniae. International Journal of Molecular Sciences. 2018; 19(6):1685. https://doi.org/10.3390/ijms19061685
Chicago/Turabian StyleBellich, Barbara, Cristina Lagatolla, Alessandro Tossi, Monica Benincasa, Paola Cescutti, and Roberto Rizzo. 2018. "Influence of Bacterial Biofilm Polysaccharide Structure on Interactions with Antimicrobial Peptides: A Study on Klebsiella pneumoniae" International Journal of Molecular Sciences 19, no. 6: 1685. https://doi.org/10.3390/ijms19061685
APA StyleBellich, B., Lagatolla, C., Tossi, A., Benincasa, M., Cescutti, P., & Rizzo, R. (2018). Influence of Bacterial Biofilm Polysaccharide Structure on Interactions with Antimicrobial Peptides: A Study on Klebsiella pneumoniae. International Journal of Molecular Sciences, 19(6), 1685. https://doi.org/10.3390/ijms19061685