N-3 Polyunsatured Fatty Acids in Menopausal Transition: A Systematic Review of Depressive and Cognitive Disorders with Accompanying Vasomotor Symptoms
Abstract
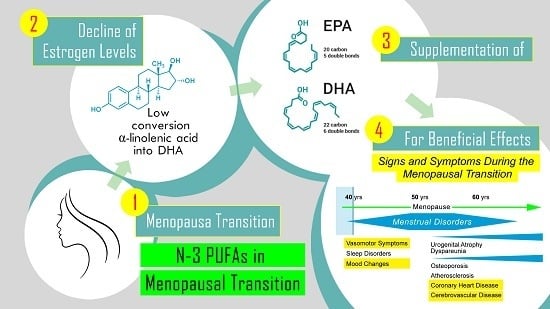
:1. Introduction
Biologic Plausibility
2. Results
2.1. N-3 LCPUFAs in Menopausal Depressive and Cognitive Symptoms
2.2. N-3 LCPUFAs in Menopausal Hot Flashes
3. Materials and Methods
4. Conclusions and Future Direction
Author Contributions
Conflicts of Interest
References
- Depression. Available online: http://www.who.int/news-room/fact-sheets/detail/depression (accessed on 22 June 2018).
- Rush, A.J.; Warden, D.; Wisniewski, S.R.; Fava, M.; Trivedi, M.H.; Gaynes, B.N.; Nierenberg, A.A. STAR*D: Revising conventional wisdom. CNS Drugs 2009, 23, 627–647. [Google Scholar] [PubMed]
- Weissman, M.M.; Leaf, P.J.; Holzer, C.E., 3rd; Myers, J.K.; Tischler, G.L. The epidemiology of depression. An update on sex differences in rates. J. Affect. Disord. 1984, 7, 179–188. [Google Scholar] [CrossRef]
- Parker, G.; Brotchie, H. Gender differences in depression. Int. Rev. Psychiatry 2010, 22, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.N. Mood disorders in midlife women: Understanding the critical window and its clinical implications. Menopause 2014, 21, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.E.; Dennerstein, L.; Finch, S.; Szoeke, C.E. Impact of menopausal status on negative mood and depressive symptoms in a longitudinal sample spanning 20 years. Menopause 2017, 24, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J.; Group, S.C. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. J. Clin. Endocrinol. Metab. 2012, 97, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.G.; Cahir, N.; Robertson, D.M.; Groome, N.P.; Dudley, E.; Green, A.; Dennerstein, L. Serum inhibins A and B fall differentially as FSH rises in perimenopausal women. Clin. Endocrinol. 1998, 48, 809–813. [Google Scholar] [CrossRef]
- Burger, H.G.; Hale, G.E.; Dennerstein, L.; Robertson, D.M. Cycle and hormone changes during perimenopause: The key role of ovarian function. Menopause 2008, 15 Pt 1, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.B.; Aggarwal, P. Estrogen and basal forebrain cholinergic neurons: Implications for brain aging and Alzheimer’s disease-related cognitive decline. Horm. Behav. 1998, 34, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Epperson, C.N.; Mathews, S.B. Menopausal Symptoms and Their Management. Endocrinol. Metab. Clin. N. Am. 2015, 44, 497–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, M.T.; Maki, P.M.; McDermott, M.P. Cognition and mood in perimenopause: A systematic review and meta-analysis. J. Steroid Biochem. Mol. Biol. 2014, 142, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feld, J.; Halbreich, U.; Karkun, S. The association of perimenopausal mood disorders with other reproductive-related disorders. CNS Spectr. 2005, 10, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Haynes, P.; Parry, B.L. Mood disorders and the reproductive cycle: Affective disorders during the menopause and premenstrual dysphoric disorder. Psychopharmacol. Bull. 1998, 34, 313–318. [Google Scholar] [PubMed]
- Schiller, C.E.; Johnson, S.L.; Abate, A.C.; Schmidt, P.J.; Rubinow, D.R. Reproductive Steroid Regulation of Mood and Behavior. Compr. Physiol. 2016, 6, 1135–1160. [Google Scholar] [PubMed]
- Santoro, N.; Epperson, C.N.; Mathews, S.B. Menopausal Symptoms and Their Management. Endocrinol. Metab. Clin. N. Am. 2015, 44, 497–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, E.W.; Sammel, M.D.; Lin, H.; Gracia, C.R.; Pien, G.W.; Nelson, D.B.; Sheng, L. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet. Gynecol. 2007, 110 Pt 1, 230–240. [Google Scholar] [CrossRef]
- Cohen, L.S.; Soares, C.N.; Vitonis, A.F.; Otto, M.W.; Harlow, B.L. Risk for new onset of depression during the menopausal transition: The Harvard study of moods and cycles. Arch. Gen. Psychiatry 2006, 63, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Sammel, M.D.; Lin, H.; Nelson, D.B. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch. Gen. Psychiatry 2006, 63, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Bromberger, J.T.; Assmann, S.F.; Avis, N.E.; Schocken, M.; Kravitz, H.M.; Cordal, A. Persistent mood symptoms in a multiethnic community cohort of pre- and perimenopausal women. Am. J. Epidemiol. 2003, 158, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Joffe, H.; Hall, J.E.; Soares, C.N.; Hennen, J.; Reilly, C.J.; Carlson, K.; Cohen, L.S. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause 2002, 9, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, B.B. Estrogenic effects on memory in women. Ann. N. Y. Acad. Sci. 1994, 743, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, B.B. Estrogen and cognitive functioning in women. Endocr. Rev. 2003, 24, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.J. Depression, the perimenopause, and estrogen therapy. Ann. N. Y. Acad. Sci. 2005, 1052, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Maki, P.M.; Freeman, E.W.; Greendale, G.A.; Henderson, V.W.; Newhouse, P.A.; Schmidt, P.J.; Scott, N.F.; Shively, C.A.; Soares, C.N. Summary of the National Institute on Aging-sponsored conference on depressive symptoms and cognitive complaints in the menopausal transition. Menopause 2010, 17, 815–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinton, R.D.; Tran, J.; Proffitt, P.; Montoya, M. 17 beta-Estradiol enhances the outgrowth and survival of neocortical neurons in culture. Neurochem. Res. 1997, 22, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Keenan, P.A.; Ezzat, W.H.; Ginsburg, K.; Moore, G.J. Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology 2001, 26, 577–590. [Google Scholar] [CrossRef]
- Lacreuse, A.; Wilson, M.E.; Herndon, J.G. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol. Aging 2002, 23, 589–600. [Google Scholar] [CrossRef]
- Rapp, P.R.; Morrison, J.H.; Roberts, J.A. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J. Neurosci. 2003, 23, 5708–5714. [Google Scholar] [CrossRef] [PubMed]
- Golub, M.S.; Germann, S.L.; Hogrefe, C.E. Endocrine disruption and cognitive function in adolescent female rhesus monkeys. Neurotoxicol. Teratol. 2004, 26, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Joffe, H.; Hall, J.E.; Gruber, S.; Sarmiento, I.A.; Cohen, L.S.; Yurgelun-Todd, D.; Martin, K.A. Estrogen therapy selectively enhances prefrontal cognitive processes: A randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause 2006, 13, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Dumas, J.; Hancur-Bucci, C.; Naylor, M.; Sites, C.; Newhouse, P. Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: Evidence for the critical period hypothesis. Horm. Behav. 2008, 53, 159–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biegon, A.; McEwen, B.S. Modulation by estradiol of serotonin receptors in brain. J. Neurosci. 1982, 2, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kugaya, A.; Epperson, C.N.; Zoghbi, S.; van Dyck, C.H.; Hou, Y.; Fujita, M.; Staley, J.K.; Garg, P.K.; Seibyl, J.P.; Innis, R.B. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am. J. Psychiatry 2003, 160, 1522–1524. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.J.; Nieman, L.; Danaceau, M.A.; Tobin, M.B.; Roca, C.A.; Murphy, J.H.; Rubinow, D.R. Estrogen replacement in perimenopause-related depression: A preliminary report. Am. J. Obstet. Gynecol. 2000, 183, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.S.; Soares, C.N.; Poitras, J.R.; Prouty, J.; Alexander, A.B.; Shifren, J.L. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: A preliminary report. Am. J. Psychiatry 2003, 160, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, B.B. Affective changes with estrogen and androgen replacement therapy in surgically menopausal women. J. Affect. Disord. 1988, 14, 177–187. [Google Scholar] [CrossRef]
- Montgomery, J.C.; Appleby, L.; Brincat, M.; Versi, E.; Tapp, A.; Fenwick, P.B.; Studd, J.W. Effect of oestrogen and testosterone implants on psychological disorders in the climacteric. Lancet 1987, 1, 297–299. [Google Scholar] [CrossRef]
- Schneider, L.S.; Small, G.W.; Hamilton, S.H.; Bystritsky, A.; Nemeroff, C.B.; Meyers, B.S. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am. J. Geriatr. Psychiatry 1997, 5, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Woods, N.F.; Mitchell, E.S.; Adams, C. Memory functioning among midlife women: Observations from the Seattle Midlife Women’s Health Study. Menopause 2000, 7, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Decsi, T.; Kennedy, K. Sex-specific differences in essential fatty acid metabolism. Am. J. Clin. Nutr. 2011, 94 (Suppl. 6), 1914S–1919S. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Thompson, R.L.; Harrison, R.A.; Summerbell, C.D.; Ness, A.R.; Moore, H.J.; Worthington, H.V.; Durrington, P.N.; Higgins, J.P.; Capps, N.E.; et al. Risks and benefits of omega-3 fats for mortality, cardiovascular disease, and cancer: Systematic review. BMJ 2006, 332, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C.; Heinrich, J.; Koletzko, B. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metabolism 2010, 59, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harslof, L.B.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Carver, J.D.; Benford, V.J.; Han, B.; Cantor, A.B. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res. Bull. 2001, 56, 79–85. [Google Scholar] [CrossRef]
- Browning, L.M.; Walker, C.G.; Mander, A.P.; West, A.L.; Madden, J.; Gambell, J.M.; Young, S.; Wang, L.; Jebb, S.A.; Calder, P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012, 96, 748–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mischoulon, D.; Freeman, M.P. Omega-3 fatty acids in psychiatry. Psychiatr. Clin. N. Am. 2013, 36, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.M.; Seguin, J.; Sieswerda, L.E. Omega-3 fatty acids as treatments for mental illness: Which disorder and which fatty acid? Lipids Health Dis. 2007, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, B.; Garland, M.R. Essential fatty acids and mental health. Br. J. Psychiatry 2005, 186, 275–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assisi, A.; Banzi, R.; Buonocore, C.; Capasso, F.; Di Muzio, V.; Michelacci, F.; Renzo, D.; Tafuri, G.; Trotta, F.; Vitocolonna, M.; et al. Fish oil and mental health: The role of n-3 long-chain polyunsaturated fatty acids in cognitive development and neurological disorders. Int. Clin. Psychopharmacol. 2006, 21, 319–336. [Google Scholar] [CrossRef] [PubMed]
- De la Presa Owens, S.; Innis, S.M. Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and alpha-linolenic acid deficient diet in formula-fed piglets. J. Nutr. 1999, 129, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Sakayori, N.; Kikkawa, T.; Tokuda, H.; Kiryu, E.; Yoshizaki, K.; Kawashima, H.; Yamada, T.; Arai, H.; Kang, J.X.; Katagiri, H.; et al. Maternal dietary imbalance between omega-6 and omega-3 polyunsaturated fatty acids impairs neocortical development via epoxy metabolites. Stem Cells 2016, 34, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Linnoila, M.; Umhau, J.C.; Rawlings, R.; George, D.T.; Salem, N., Jr. Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late-onset alcoholics. Biol. Psychiatry 1998, 44, 235–242. [Google Scholar] [CrossRef]
- Chalon, S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Carlezon, W.A., Jr.; Mague, S.D.; Parow, A.M.; Stoll, A.L.; Cohen, B.M.; Renshaw, P.F. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol. Psychiatry 2005, 57, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Hoffmire, C.A.; Block, R.C.; Thevenet-Morrison, K.; van Wijngaarden, E. Associations between omega-3 poly-unsaturated fatty acids from fish consumption and severity of depressive symptoms: An analysis of the 2005–2008 National Health and Nutrition Examination Survey. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beydoun, M.A.; Fanelli Kuczmarski, M.T.; Beydoun, H.A.; Hibbeln, J.R.; Evans, M.K.; Zonderman, A.B. omega-3 fatty acid intakes are inversely related to elevated depressive symptoms among United States women. J. Nutr. 2013, 143, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.H.; Hannestad, J. Omega-3 fatty acids for the treatment of depression: Systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.J.; Fallon, E.M.; Kalish, B.T.; Gura, K.M.; Puder, M. The role of the omega-3 fatty acid DHA in the human life cycle. J. Parenter. Enter. Nutr. 2013, 37, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ciappolino, V.; Delvecchio, G.; Agostoni, C.; Mazzocchi, A.; Altamura, A.C.; Brambilla, P. The role of n-3 polyunsaturated fatty acids (n-3PUFAs) in affective disorders. J. Affect. Disord. 2017, 224, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.D.; Voineskos, A.N.; Szeszko, P.R.; Lett, T.A.; DeRosse, P.; Guha, S.; Karlsgodt, K.H.; Ikuta, T.; Felsky, D.; John, M.; et al. Brain white matter development is associated with a human-specific haplotype increasing the synthesis of long chain fatty acids. J. Neurosci. 2014, 34, 6367–6376. [Google Scholar] [CrossRef] [PubMed]
- Chhetry, B.T.; Hezghia, A.; Miller, J.M.; Lee, S.; Rubin-Falcone, H.; Cooper, T.B.; Oquendo, M.A.; Mann, J.J.; Sublette, M.E. Omega-3 polyunsaturated fatty acid supplementation and white matter changes in major depression. J. Psychiatr. Res. 2016, 75, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Villegas, A.; Henriquez, P.; Figueiras, A.; Ortuno, F.; Lahortiga, F.; Martinez-Gonzalez, M.A. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. Eur. J. Nutr. 2007, 46, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Timonen, M.; Horrobin, D.; Jokelainen, J.; Laitinen, J.; Herva, A.; Rasanen, P. Fish consumption and depression: The Northern Finland 1966 birth cohort study. J. Affect. Disord. 2004, 82, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, L.A.; He, K.; Whooley, M.A.; Daviglus, M.L.; Liu, K. Higher dietary intake of long-chain omega-3 polyunsaturated fatty acids is inversely associated with depressive symptoms in women. Nutrition 2009, 25, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Giltay, E.J.; Gooren, L.J.; Toorians, A.W.; Katan, M.B.; Zock, P.L. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am. J. Clin. Nutr. 2004, 80, 1167–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graziottin, A.; Serafini, A. Depression and the menopause: Why antidepressants are not enough? Menopause Int. 2009, 15, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.; Hirsch, C.; Whitmer, R.; Cauley, J.; Harris, T. The association of bone mineral density and depression in an older population. J. Am. Geriatr. Soc. 2001, 49, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, T.; Reis, S.E.; Olson, M.; Owens, J.; Kelsey, S.F.; Pepine, C.J.; Reichek, N.; Rogers, W.J.; Merz, C.N.; Sopko, G.; et al. Psychosocial variables are associated with atherosclerosis risk factors among women with chest pain: The WISE study. Psychosom. Med. 2001, 63, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Asselin, G.; Merette, C.; Poulin, M.J.; Dodin, S. Ethyl-eicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: A double-blind, placebo-controlled, randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.P.; Hibbeln, J.R.; Silver, M.; Hirschberg, A.M.; Wang, B.; Yule, A.M.; Petrillo, L.F.; Pascuillo, E.; Economou, N.I.; Joffe, H.; et al. Omega-3 fatty acids for major depressive disorder associated with the menopausal transition: A preliminary open trial. Menopause 2011, 18, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.S.; Joffe, H.; Guthrie, K.A.; Ensrud, K.E.; Freeman, M.; Carpenter, J.S.; Learman, L.A.; Newton, K.M.; Reed, S.D.; Manson, J.E.; et al. Efficacy of omega-3 for vasomotor symptoms treatment: A randomized controlled trial. Menopause 2014, 21, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, S.Z.; Kazemi, F.; Tavakolian, S.; Rahimi, A.; Oshvandi, K.; Soltanian, A.; Shobeiri, F. Effect of Citalopram in Combination with Omega-3 on Depression in Post-menopausal Women: A Triple Blind Randomized Controlled Trial. J. Clin. Diagn. Res. 2016, 10, QC01–QC05. [Google Scholar] [CrossRef] [PubMed]
- Strike, S.C.; Carlisle, A.; Gibson, E.L.; Dyall, S.C. A High Omega-3 Fatty Acid Multinutrient Supplement Benefits Cognition and Mobility in Older Women: A Randomized, Double-blind, Placebo-controlled Pilot Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.H.; Troesch, B.; Weber, P. Inadequate supply of vitamins and DHA in the elderly: Implications for brain aging and Alzheimer-type dementia. Nutrition 2015, 31, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, M.F.; Ryan, C.M.; Sheu, L.; Yao, J.K.; Conklin, S.M.; Manuck, S.B. Serum phospholipid docosahexaenonic acid is associated with cognitive functioning during middle adulthood. J. Nutr. 2010, 140, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Yurko-Mauro, K.; McCarthy, D.; Rom, D.; Nelson, E.B.; Ryan, A.S.; Blackwell, A.; Salem, N., Jr.; Stedman, M.; Investigators, M. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010, 6, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Su, K.P.; Cheng, T.C.; Liu, H.C.; Chang, C.J.; Dewey, M.E.; Stewart, R.; Huang, S.Y. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R., Jr.; Weiner, M.; et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Stearns, V.; Ullmer, L.; Lopez, J.F.; Smith, Y.; Isaacs, C.; Hayes, D. Hot flushes. Lancet 2002, 360, 1851–1861. [Google Scholar] [CrossRef]
- Guthrie, K.A.; LaCroix, A.Z.; Ensrud, K.E.; Joffe, H.; Newton, K.M.; Reed, S.D.; Caan, B.; Carpenter, J.S.; Cohen, L.S.; Freeman, E.W.; et al. Pooled Analysis of Six Pharmacologic and Nonpharmacologic Interventions for Vasomotor Symptoms. Obstet. Gynecol. 2015, 126, 413–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, S.D.; Guthrie, K.A.; Newton, K.M.; Anderson, G.L.; Booth-LaForce, C.; Caan, B.; Carpenter, J.S.; Cohen, L.S.; Dunn, A.L.; Ensrud, K.E.; et al. Menopausal quality of life: RCT of yoga, exercise, and omega-3 supplements. Am. J. Obstet. Gynecol. 2014, 210, 244.e1–244.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Depressive and Cognitive Symptoms | n Sample | N-PUFA Assessed Daily Amounts | Duration (Weeks) | Outcome Measures | Major Finding |
|---|---|---|---|---|---|
| Study | |||||
| [72] | 19 postmenopausal women | Lovaza 2 g/day (1-g capsule: 465 mg EPA + 375 mg DHA + 160 mg small amounts of other omega-3 fatty acids) | 8 weeks | The primary outcome was change in depressive symptoms from beginning to end of the study, as measured by MADRS | The depressive symptoms improved with treatment with a significant decrease in MADRS scores |
| [71] | 120 post-menopausal women omega-3s (n = 59) or placebo (n = 61) | A 500-mg capsule three times daily (350 mg of EPA and 50 mg of DHA in the form of ethyl ester) | 8 weeks | Primary outcome was to compare enriched (E-EPA) supplementation with placebo for the treatment of PD measured by (PGWB) and depressive symptoms measured by HSCL-D-20 and HAM-D-21 | Supplementation with E-EPA omega-3 fatty acid improved significantly more than placebo in women with PD without MDE at baseline, but not significantly in women with PD and with MDE |
| [73] | 355 post-menopausal women were randomly assigned to receive omega-3s (n = 177) or placebo (n = 178) | 1.8 g/day of omega-3 supplementation (3 pills/day, each containing 425 mg of EPA, 100 mg DHA and 90 mg of other omega-3s) | 12 weeks | The secondary outcomes were sleep quality measured by (PSQI), insomnia symptoms measured by (ISI), depressive symptoms measured by (PHQ-8), and anxiety measured by (GAD-7) | Omega-3s did not significantly reduce sleep or mood compared to placebo |
| [74] | 60 postmenopausal women: n = 30 citalopram + 1 g omega-3s and n = 30 citalopram + placebo | 1 g/day of omega-3 fish oil capsules | 4 weeks | The effect of a combination of omega-3 and citalopram in the treatment of women with post-menopausal depression measured by BDI | Omega-3s in combination with citalopram demonstrated to reduce the severity of depression in post-menopausal wome |
| [75] | 27 post-menopausal women: n = 15 received multinutrient supplement) and n = 12 received placebo | Four capsules per day of Efalex Active 50+, corresponding to: 1 g DHA, 160 mg eicosapentaenoic acid, 240 mg Ginkgo biloba, 60 mg phosphatidyl-serine, 20 mg d-α tocopherol, 1 mg folic acid, and 20 μg vitamin B12 | 24 weeks | The primary outcome measures were based on changes in mobility (including Habitual walking (HW), fast walking (FW), and Vertical jump height (VJH) and cognition (including psychomotor response latency (MOT), Verbal Recognition Memory (VRM), and paired associate learning (PAL)) | Multinutrient supplement containing high doses of DHA and eicosapentaenoic acid significantly improves some cognition and mobility measures in post-menopausal women |
| Hot Flashes and Vasomotor Symptoms | n Sample | N-PUFA Assessed Daily Amounts | Duration (Weeks) | Outcome Measures | Major Finding |
|---|---|---|---|---|---|
| Study | |||||
| [72] | 19 women | Lovaza 2 g/day (1 g capsule: 465 mg EPA + 375 mg DHA + 160 mg small amounts of other omega-3 fatty acids) | 8 weeks | The secondary outcome was change in HF from beginning to end of the study, as measured by hot flash diary and HFRDIS scores | HF improved significantly with treatment, as evident in hot flash diary scores and HFRDIS scores |
| [71] | E-EPA, n = 43; placebo, n = 39 | A 500-mg capsule three times daily (350 mg of EPA and 50 mg of DHA in the form of ethyl ester) | 8 weeks | Secondary objectives were to compare the mean change in HFs (frequency, intensity, and score) and the proportion of HF responders (≥50% reduction in HF frequency between baseline and week 8) | Supplementation with E-EPA omega-3 fatty acid reduced HF frequency and improved the HF score relative to placebo |
| [73] | 355 women were randomly assigned to receive omega-3s (n = 177) or placebo (n = 178) | 1.8 g/day of omega-3 supplementation (3 pills/day, each containing 425 mg of EPA, 100 mg DHA and 90 mg of other omega-3s) | 12 weeks | The primary outcomes were VMS frequency and bother based on daily diaries at baseline and weeks 6 and 12 | Omega-3s did not significantly reduce hot flash frequency compared to placebo (p = 0.28) |
| [82] | 177 women to omega-3 and 178 to placebo | 1.8 g/day of omega-3 fish oil capsules (425 mg E-EPA acid, 100 mg DHA and 90 mg of other omega-3s three times a day) | 12 weeks | The MsFLASH Network, has conducted three large RCTs for treatment of menopausal VMS testing six interventions including omega-3 fatty acid supplementation | The MsFLASH 02 interventions of yoga, exercise, and omega-3 showed little effect in reducing vasomotor symptom frequency or bother relative to control |
| [83] | 355 women | 1.8 g/day of omega-3 (425 mg E-EPA, 100 mg DHA and 90 mg of other omega-3s) | 12 weeks | MENQOL total and domain (VMS, psychosocial, physical and sexual) scores | Hot flash interference, stress, pain and sexual function showed no improvement with exercise or omega-3 interventions over usual care or placebo, respectively |
| Menopausal Symptoms | Positive Results | Negative Results | Positive Results without Statistical Significance |
|---|---|---|---|
| Hot flashes | [71,82] | [73,83] | [72] |
| Depressive symptoms | [74] | [73] | [71,72] |
| Cognitive symptoms | [75] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciappolino, V.; Mazzocchi, A.; Enrico, P.; Syrén, M.-L.; Delvecchio, G.; Agostoni, C.; Brambilla, P. N-3 Polyunsatured Fatty Acids in Menopausal Transition: A Systematic Review of Depressive and Cognitive Disorders with Accompanying Vasomotor Symptoms. Int. J. Mol. Sci. 2018, 19, 1849. https://doi.org/10.3390/ijms19071849
Ciappolino V, Mazzocchi A, Enrico P, Syrén M-L, Delvecchio G, Agostoni C, Brambilla P. N-3 Polyunsatured Fatty Acids in Menopausal Transition: A Systematic Review of Depressive and Cognitive Disorders with Accompanying Vasomotor Symptoms. International Journal of Molecular Sciences. 2018; 19(7):1849. https://doi.org/10.3390/ijms19071849
Chicago/Turabian StyleCiappolino, Valentina, Alessandra Mazzocchi, Paolo Enrico, Marie-Louise Syrén, Giuseppe Delvecchio, Carlo Agostoni, and Paolo Brambilla. 2018. "N-3 Polyunsatured Fatty Acids in Menopausal Transition: A Systematic Review of Depressive and Cognitive Disorders with Accompanying Vasomotor Symptoms" International Journal of Molecular Sciences 19, no. 7: 1849. https://doi.org/10.3390/ijms19071849
APA StyleCiappolino, V., Mazzocchi, A., Enrico, P., Syrén, M. -L., Delvecchio, G., Agostoni, C., & Brambilla, P. (2018). N-3 Polyunsatured Fatty Acids in Menopausal Transition: A Systematic Review of Depressive and Cognitive Disorders with Accompanying Vasomotor Symptoms. International Journal of Molecular Sciences, 19(7), 1849. https://doi.org/10.3390/ijms19071849