A hnRNP K–AR-Related Signature Reflects Progression toward Castration-Resistant Prostate Cancer
Abstract
:1. Introduction
2. Results
2.1. Role of hnRNP K in ADT Resistance
2.2. Role of hnRNP K Phosphorylation in ADT Resistance
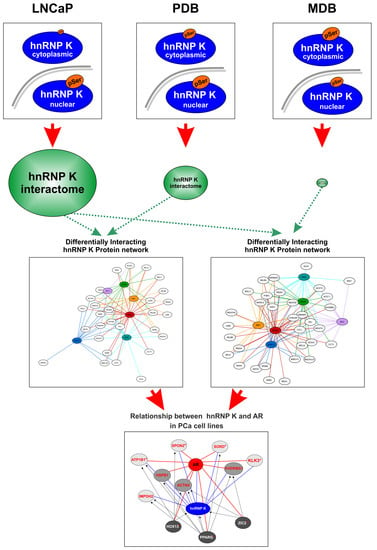
2.3. Characterization of the hnRNP K Interactome in LNCaP, PDB, and MDB Cell Lines
2.4. Signature Identification for Potential PCa and CRPC Patient Stratification
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Fractionation
4.3. 1D and 2D Gel Electrophoresis and WB Analyses
4.4. hnRNP K Silencing and Reporter Assay
4.5. Co-Immunoprecipitation
4.6. MS Analysis
4.7. Network Analysis
4.8. Differential Expression and Enrichment Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADT | Androgen deprivation therapy |
| AR | Androgen receptor |
| ARG | Androgen-responsive gene |
| BIC | Bicalutamide |
| Co-IP | Co-immunoprecipitate |
| CRPC | Castration-resistant prostate cancer |
| DAVID | Database for Annotation Visualization and Integrated Discovery |
| DEP | Differentially expressed protein |
| DIhKP | Differentially interacting hnRNP K protein |
| DHT | 5-α-dihydrotestosterone |
| GO | Gene ontology |
| hK-TF | Transcription factor potentially regulating the expression of differentially interacting hnRNP K protein |
| LTQ | Linear trap quadrupole |
| MS | Mass spectrometry |
| PTM | Post-translation modifications |
| TF | Transcription factor |
| TFBS | Transcription factor binding sites |
| PAGE | Polyacrylamide gel electrophoresis |
| PCa | Prostate cancer |
| WB | Western blotting |
References
- Katsogiannou, M.; Ziouziou, H.; Karaki, S.; Andrieu, C.; Henry de Villeneuve, M.; Rocchi, P. The hallmarks of castration-resistant prostate cancers. Cancer Treat. Rev. 2015, 41, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Cattrini, C.; Zanardi, E.; Vallome, G.; Cavo, A.; Cerbone, L.; Di Meglio, A.; Fabbroni, C.; Latocca, M.M.; Rizzo, F.; Messina, C.; et al. Targeting androgen-independent pathways: New chances for patients with prostate cancer? Crit. Rev. Oncol. Hematol. 2017, 118, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Zarif, J.C.; Miranti, C.K. The importance of non-nuclear AR signaling in prostate cancer progression and therapeutic resistance. Cell Signal. 2016, 28, 348–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, R.B.; Mehra, R.; Chinnaiyan, A.M.; Shen, R.; Ghosh, D.; Zhou, M.; Macvicar, G.R.; Varambally, S.; Harwood, J.; Bismar, T.A.; et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: Lessons from a rapid autopsy program. Cancer Res. 2004, 64, 9209–9216. [Google Scholar] [CrossRef] [PubMed]
- Beltran, H.; Yelensky, R.; Frampton, G.M.; Park, K.; Downing, S.R.; MacDonald, T.Y.; Jarosz, M.; Lipson, D.; Tagawa, S.T.; Nanus, D.M.; et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur. Urol. 2012, 63, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, C.; Massari, F.; Iacovelli, R.; Fiorentino, M.; Montironi, R.; Di Nunno, V.; Giunchi, F.; Brunelli, M.; Tortora, G. Prostate cancer heterogeneity: Discovering novel molecular targets for therapy. Cancer Treat. Rev. 2017, 54, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, A.W.; Gleave, M.E. Targeting the adaptive molecular landscape of castration-resistant prostate cancer. EMBO Mol. Med. 2015, 7, 878–894. [Google Scholar] [CrossRef] [PubMed]
- Emmons, M.F.; Faiao-Flores, F.; Smalley, K.S. The role of phenotypic plasticity in the escape of cancer cells from targeted therapy. Biochem. Pharmacol. 2016, 122, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Kumari, S.; Hu, Q.; Senapati, D.; Venkadakrishnan, V.B.; Wang, D.; DePriest, A.D.; Schlanger, S.E.; Ben-Salem, S.; Valenzuela, M.M.; et al. A comprehensive analysis of coregulator recruitment, androgen receptor function and gene expression in prostate cancer. Elife 2017, 6, e28482. [Google Scholar] [CrossRef] [PubMed]
- Bomsztyk, K.; Denisenko, O.; Ostrowski, J. hnRNP K: One protein multiple processes. Bioessays 2004, 26, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Mikula, M.; Ostrowski, J. Heterogeneous Nuclear Ribonucleoprotein K—Hnrnp K. The Transcription Factor Encyclopedia. Genome Biol. 2012, 13, R24. Available online: http://www.cisreg.ca/cgi-bin/tfe/articles.pl?tfid=551 (accessed on 27 November 2017).
- Barboro, P.; Ferrari, N.; Balbi, C. Emerging roles of heterogeneous nuclear ribonucleoprotein K (hnRNP K) in cancer progression. Cancer Lett. 2014, 352, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Barboro, P.; Repaci, E.; Rubagotti, A.; Salvi, S.; Boccardo, S.; Spina, B.; Truini, M.; Introini, C.; Puppo, P.; Ferrari, N.; et al. Heterogeneous nuclear ribonucleoprotein K: Altered pattern of expression associated with diagnosis and prognosis of prostate cancer. Br. J. Cancer 2009, 100, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Barboro, P.; Salvi, S.; Rubagotti, A.; Boccardo, S.; Spina, B.; Truini, M.; Carmignani, G.; Introini, C.; Ferrari, N.; Boccardo, F.; et al. Prostate cancer: Prognostic significance of the association of heterogeneous nuclear ribonucleoprotein K and androgen receptor expression. Int. J. Oncol. 2014, 44, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, N.K.; Kim, J.; Cinar, B.; Ramachandran, A.; Hager, M.H.; Di Vizio, D.; Adam, R.M.; Rubin, M.A.; Raychaudhuri, P.; De Benedetti, A.; et al. Heterogeneous nuclear ribonucleoprotein K is a novel regulator of androgen receptor translation. Cancer Res. 2009, 69, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Barboro, P.; Repaci, E.; Ferrari, N.; Rubagotti, A.; Boccardo, F.; Balbi, C. Androgen receptor and heterogeneous nuclear ribonucleoprotein K colocalize in the nucleoplasm and are modulated by bicalutamide and 4-hydroxy-tamoxifen in prostatic cancer cell lines. Prostate 2011, 71, 1466–1479. [Google Scholar] [CrossRef] [PubMed]
- Ciarlo, M.; Benelli, R.; Barbieri, O.; Minghelli, S.; Barboro, P.; Balbi, C.; Ferrari, N. Regulation of neuroendocrine differentiation by AKT/hnRNPK/AR/beta-catenin signaling in prostate cancer cells. Int. J. Cancer 2012, 131, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.; Balk, S.P. Reprogramming to resist. Science 2017, 355, 29–30. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, N.; Granata, I.; Capaia, M.; Piccirillo, M.; Guarracino, M.R.; Vene, R.; Brizzolara, A.; Petretto, A.; Inglese, E.; Morini, M.; et al. Adaptive phenotype drives resistance to androgen deprivation therapy in prostate cancer. Cell Commun. Signal. 2017, 15, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barboro, P.; Borzi, L.; Repaci, E.; Ferrari, N.; Balbi, C. Androgen receptor activity is affected by both nuclear matrix localization and the phosphorylation status of the heterogeneous nuclear ribonucleoprotein K in anti-androgen-treated LNCaP cells. PLoS ONE 2013, 8, e79212. [Google Scholar] [CrossRef] [PubMed]
- Moumen, A.; Magill, C.; Dry, K.L.; Jackson, S.P. ATM-dependent phosphorylation of heterogeneous nuclear ribonucleoprotein K promotes p53 transcriptional activation in response to DNA damage. Cell Cycle 2013, 12, 698–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habelhah, H.; Shah, K.; Huang, L.; Burlingame, A.L.; Shokat, K.M.; Ronai, Z. Identification of new JNK substrate using ATP pocket mutant JNK and a corresponding ATP analogue. J. Biol. Chem. 2001, 276, 18090–18095. [Google Scholar] [CrossRef] [PubMed]
- Laury-Kleintop, L.D.; Tresini, M. Hammond, O. Compartmentalization of hnRNP-K during cell cycle progression and its interaction with calponin in the cytoplasm. J. Cell. Biochem. 2005, 95, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Habelhah, H.; Shah, K.; Huang, L.; Ostareck-Lederer, A.; Burlingame, A.L.; Shokat, K.M.; Hentze, M.W.; Ronai, Z. ERK phosphorylation drives cytoplasmic accumulation of hnRNP-K and inhibition of mRNA translation. Nat. Cell Biol. 2001, 3, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Sampson, N.; Neuwirt, H.; Puhr, M.; Klocker, H.; Eder, I.E. In vitro model systems to study androgen receptor signaling in prostate cancer. Endocr. Relat. Cancer 2013, 20, R49–R64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, Y.; Nagata, K.; Suzuki, N.; Yokoyama, R.; Yamanaka, Y.; Kitamura, H.; Hirano, H.; Ohara, O. Characterization of multiple alternative forms of heterogeneous nuclear ribonucleoprotein K by phosphate-affinity electrophoresis. Proteomics 2010, 10, 3884–3895. [Google Scholar] [CrossRef] [PubMed]
- Mikula, M.; Dzwonek, A.; Karczmarski, J.; Rubel, T.; Dadlez, M.; Wyrwicz, L.S.; Bomsztyk, K.; Ostrowski, J. Landscape of the hnRNP K protein-protein interactome. Proteomics 2006, 6, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Winterhalter, C.; Widera, P.; Krasnogor, N. JEPETTO: A Cytoscape plugin for gene set enrichment and topological analysis based on interaction networks. Bioinformatics 2013, 30, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.L.; Massie, C.E.; Ramos-Montoya, A.; Zecchini, V.; Scott, H.E.; Lamb, A.D.; MacArthur, S.; Stark, R.; Warren, A.Y.; Mills, I.G.; et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell 2013, 23, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Oh, K.J.; Park, R.Y.; Xuan, N.T.; Kang, T.W.; Kwon, D.D.; Choi, C.; Kim, M.S.; Nam, K.I.; Ahn, K.Y.; et al. HOXB13 promotes androgen independent growth of LNCaP prostate cancer cells by the activation of E2F signaling. Mol. Cancer 2010, 9, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Cai, Y.; Liu, B.; Jiao, X.; Li, Z.T.; Guo, D.Y.; Li, X.W.; Wang, Y.J.; Yang, D.K. HOXA13 is associated with unfavorable survival and acts as a novel oncogene in prostate carcinoma. Future Oncol. 2017, 13, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chang, W.; Yang, G.; Ren, C.; Park, S.; Karantanos, T.; Karanika, S.; Wang, J.; Yin, J.; Shah, P.K.; et al. Targeting poly(ADP-ribose) polymerase and the c-Myb-regulated DNA damage response pathway in castration-resistant prostate cancer. Sci. Signal. 2015, 7, ra47. [Google Scholar] [CrossRef] [PubMed]
- Alghadi, A.Y.; Khalil, A.M.; Alazab, R.S.; Aldaoud, N.H.; Zyoud, A.M.-S. Unique Expression of the XBP1 Gene Correlates with with Human Prostate Cancer. J. Investig. Genom. 2017, 4, 00091. [Google Scholar] [CrossRef]
- Olokpa, E.; Moss, P.E.; Stewart, L.V. Crosstalk between the Androgen Receptor and PPAR Gamma Signaling Pathways in the Prostate. PPAR Res. 2017, 2017, 9456020. [Google Scholar] [CrossRef] [PubMed]
- Mendiratta, P.; Mostaghel, E.; Guinney, J.; Tewari, A.K.; Porrello, A.; Barry, W.T.; Nelson, P.S.; Febbo, P.G. Genomic strategy for targeting therapy in castration-resistant prostate cancer. J. Clin. Oncol. 2009, 27, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Tien, A.H.; Saladar, M.D. Androgen-Responsive Gene Expression in Prostate Cancer Progression, 1st ed.; Springer: New York, NY, USA, 2013; pp. 135–153. ISBN 978-1-4614-6182-1. [Google Scholar]
- Beitel, L. Androgen Receptor Interacting Proteins and Coregulators Table. 2010. Available online: http://androgendb.mcgill.ca/ARinteract.pdf (accessed on 5 December 2017).
- Larkin, S.E.; Zeidan, B.; Taylor, M.G.; Bickers, B.; Al-Ruwaili, J.; Aukim-Hastie, C.; Townsend, P.A. Proteomics in prostate cancer biomarker discovery. Expert Rev. Proteom. 2018, 7, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Prensner, J.R.; Rubin, M.A.; Wei, J.T.; Chinnaiyan, A.M. Beyond PSA: The next generation of prostate cancer biomarkers. Sci. Transl. Med. 2012, 4, 127rv3. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S.; Yoneyama, T.; Tobisawa, Y.; Ohyama, C. Recent progress and perspectives on prostate cancer biomarkers. Int. J. Clin. Oncol. 2017, 22, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Intasqui, P.; Bertolla, R.P.; Sadi, M.V. Prostate cancer proteomics: Clinically useful protein biomarkers and future perspectives. Expert Rev. Proteom. 2017, 15, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Z.; Hamalainen, J.; Loikkanen, I.; Moilanen, A.M.; Hirvikoski, P.; Vaisanen, T.; Paavonen, T.K.; Vaarala, M.H. Sorbitol dehydrogenase expression is regulated by androgens in the human prostate. Oncol. Rep. 2010, 23, 1233–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, X.; Li, C.; Pang, B.; Xue, M.; Wang, J.; Zhou, J. Spondin-2 (SPON2), a more prostate-cancer-specific diagnostic biomarker. PLoS ONE 2012, 7, e37225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.P.; Guo, Z.Q.; Lin, L.; Liu, Q. Serum BSP, PSADT, and Spondin-2 levels in prostate cancer and the diagnostic significance of their ROC curves in bone metastasis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 61–67. [Google Scholar] [PubMed]
- Vainio, P.; Wolf, M.; Edgren, H.; He, T.; Kohonen, P.; Mpindi, J.P.; Smit, F.; Verhaegh, G.; Schalken, J.; Perala, M.; et al. Integrative genomic, transcriptomic, and RNAi analysis indicates a potential oncogenic role for FAM110B in castration-resistant prostate cancer. Prostate 2011, 72, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Honda, K. The biological role of actinin-4 (ACTN4) in malignant phenotypes of cancer. Cell Biosci. 2015, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Jasavala, R.; Martinez, H.; Thumar, J.; Andaya, A.; Gingras, A.C.; Eng, J.K.; Aebersold, R.; Han, D.K.; Wright, M.E. Identification of putative androgen receptor interaction protein modules: Cytoskeleton and endosomes modulate androgen receptor signaling in prostate cancer cells. Mol. Cell Proteom. 2007, 6, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, T.; Bishop, J.L.; Shiota, M.; Nip, K.M.; Thaper, D.; Vahid, S.; Heroux, D.; Gleave, M.; Zoubeidi, A. Hsp27 regulates EGF/beta-catenin mediated epithelial to mesenchymal transition in prostate cancer. Int. J. Cancer 2015, 136, E496–E507. [Google Scholar] [CrossRef] [PubMed]
- Zoubeidi, A.; Zardan, A.; Beraldi, E.; Fazli, L.; Sowery, R.; Rennie, P.; Nelson, C.; Gleave, M. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 2007, 67, 10455–10465. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, P.; Beraldi, E.; Ettinger, S.; Fazli, L.; Vessella, R.L.; Nelson, C.; Gleave, M. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res. 2005, 65, 11083–11093. [Google Scholar] [CrossRef] [PubMed]
- Stockley, J.; Markert, E.; Zhou, Y.; Robson, C.N.; Elliott, D.J.; Lindberg, J.; Leung, H.Y.; Rajan, P. The RNA-binding protein Sam68 regulates expression and transcription function of the androgen receptor splice variant AR-V7. Sci. Rep. 2015, 5, 13426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisone, P.; Pradella, D.; Di Matteo, A.; Belloni, E.; Ghigna, C.; Paronetto, M.P. SAM68: Signal Transduction and RNA Metabolism in Human Cancer. Biomed Res. Int. 2015, 2015, 528954. [Google Scholar] [CrossRef] [PubMed]
- Busa, R.; Paronetto, M.P.; Farini, D.; Pierantozzi, E.; Botti, F.; Angelini, D.F.; Attisani, F.; Vespasiani, G.; Sette, C. The RNA-binding protein Sam68 contributes to proliferation and survival of human prostate cancer cells. Oncogene 2007, 26, 4372–4382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Xia, D.; Zhu, J.; Chen, Y.; Chen, G.; Mo, R.; Zeng, Y.; Dai, Q.; He, H.; Liang, Y.; et al. Enhanced expression of IMPDH2 promotes metastasis and advanced tumor progression in patients with prostate cancer. Clin. Transl. Oncol. 2014, 16, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Albitar, M.; Ma, W.; Lund, L.; Albitar, F.; Diep, K.; Fritsche, H.A.; Shore, N. Predicting Prostate Biopsy Results Using a Panel of Plasma and Urine Biomarkers Combined in a Scoring System. J. Cancer 2015, 7, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Brizzolara, A.; Benelli, R.; Vene, R.; Barboro, P.; Poggi, A.; Tosetti, F.; Ferrari, N. The ErbB family and androgen receptor signaling are targets of Celecoxib in prostate cancer. Cancer Lett. 2017, 400, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.G.; Johnson, E.M.; Kinoshita, Y.; Babb, J.S.; Buckley, M.T.; Liebes, L.F.; Melamed, J.; Liu, X.M.; Kurek, R.; Ossowski, L.; et al. Androgen receptor overexpression in prostate cancer linked to Pur alpha loss from a novel repressor complex. Cancer Res. 2008, 68, 2678–2688. [Google Scholar] [CrossRef] [PubMed]
- Hang, S.P.; Tang, Y.H.; Smith, R. Functional diversity of hnRNPs: Past, present and perspectives. Biochem. J. 2010, 430, 379–392. [Google Scholar] [CrossRef]
- Rahman-Roblick, R.; Roblick, U.J.; Hellman, U.; Conrotto, P.; Liu, T.; Becker, S.; Hirschberg, D.; Jörnvall, H.; Auer, G.; Wiman, K.G. p53 targets identified by protein expression profiling. Proc. Natl. Acad. Sci. USA 2007, 104, 5401–5406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.Y.H.; Huang, S.M.; Liu, S.T.; Huang, C.H. The transactivation domain of heterogeneous nuclear ribonucleoprotein K overlaps its nuclear shuttling domain. Int. J. Biochem. Cell Biol. 2008, 40, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Vlachostergios, P.J.; Puca, L.; Beltran, H. Emerging Variants of Castration-Resistant Prostate Cancer. Curr. Oncol. Rep. 2017, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Lamb, A.D.; Massie, C.E.; Neal, D.E. The transcriptional programme of the androgen receptor (AR) in prostate cancer. BJU Int. 2014, 113, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzbeierlein, J.; Lal, P.; LaTulippe, E.; Smith, A.; Satagopan, J.; Zhang, L.; Ryan, C.; Smith, S.; Scher, H.; Scardino, P.; et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am. J. Pathol. 2004, 164, 217–227. [Google Scholar] [CrossRef]
- Coutinho, I.; Day, T.K.; Tilley, W.D.; Selth, L.A. Androgen receptor signaling in castration-resistant prostate cancer: A lesson in persistence. Endocr. Relat. Cancer 2016, 23, T179–T197. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Yokomizo, A.; Fujimoto, N.; Naito, S. Androgen receptor cofactors in prostate cancer: Potential therapeutic targets of castration-resistant prostate cancer. Curr. Cancer Drug Targets 2011, 11, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z. Androgen Receptor Coactivators in Regulation of Growth and Differentiation in Prostate Cancer. J. Cell. Physiol. 2015, 231, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Obinata, D.; Takayama, K.; Takahashi, S.; Inoue, S. Crosstalk of the Androgen Receptor with Transcriptional Collaborators: Potential Therapeutic Targets for Castration-Resistant Prostate Cancer. Cancers 2017, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Moss, P.E.; Lyles, B.E.; Stewart, L.V. The PPARgamma ligand ciglitazone regulates androgen receptor activation differently in androgen-dependent versus androgen-independent human prostate cancer cells. Exp. Cell Res. 2010, 316, 3478–3488. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.P.; Reddy, T.R.; Truong, K.T.; Suhasini, M.; Wong-Staal, F. Functional interaction of Sam68 and heterogeneous nuclear ribonucleoprotein K. Oncogene 2002, 21, 7187–7194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.S.; Smoot, M.; Cerami, E.; Kuchinsky, A.; Landys, N.; Workman, C.; Christmas, R.; Avila-Campilo, I.; Creech, M.; Gross, B.; et al. Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2007, 2, 2366–2382. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Romero, P. Pre-processing and differential expression analysis of Agilent microRNA arrays using the AgiMicroRna Bioconductor library. BMC Genom. 2011, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Meltzer, P.S. GEOquery: A bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 2007, 23, 1846–1847. [Google Scholar] [CrossRef] [PubMed]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]






| Cell Line | LNCaP(AR-FL 1) | PDB(AR-FL) | MDB(AR-FL) | 22Rv1 (ARv7 2) | PC3(AR-Null 3) |
|---|---|---|---|---|---|
| AR Status | Active | Hypersensitive | Inactive | Androgen Independent | Not Detected |
| Nucleus | |||||
| Alternatively spliced | |||||
| isoform 1 (%) | 50.1 | 66.7 | 62.5 | 76.6 | 66.8 |
| isoform 2 (%) | 49.9 | 33.3 | 37.5 | 23.4 | 33.2 |
| Phosphorylated forms | |||||
| pS116 (%) | 17.1 | 19.5 | 15.0 | 1.7 | 0.7 |
| pS284 (%) | 37.6 | 45.8 | 23.2 | 36.1 | 22.8 |
| pS353 (%) | 21.6 | 8.4 | 16.2 | 1.8 | 10.6 |
| Non-phosphorylated forms (%) | 23.6 | 26.2 | 45.5 | 60.3 | 65.8 |
| Cytoplasm | |||||
| Alternatively spliced | |||||
| isoform 1 (%) | 38.7 | 45.1 | 66.1 | 63.7 | 76.2 |
| isoform 2 (%) | 61.3 | 54.9 | 33.9 | 36.3 | 23.8 |
| Phosphorylated forms | |||||
| pS116 (%) | 0.0 | 8.2 | 9.9 | 13.3 | 6.8 |
| pS284 (%) | 0.0 | 0.0 | 42.0 | 41.3 | 0.0 |
| pS353 (%) | 24.9 | 35.7 | 21.5 | 17.8 | 23.8 |
| Non-phosphorylated forms (%) | 75.1 | 56.1 | 26.6 | 27.7 | 69.4 |
| Gene symbol/Protein Name | Differential hnRNP K Interaction | Associated hK-TF | Association with AR | Human Prostate Tissues Differential Expression | Association with PCa (Literature Evidences) | |||
|---|---|---|---|---|---|---|---|---|
| Proteomic Studies | Transcriptomic Studies | |||||||
| KLK3/PSA | MDB&PDB vs. LNCaP ↓ * | PAX5 | AR controlled | PCa vs. BPH ↓ | PCa & mCRPC vs. NT ↑ | mCRPC vs. PCa ↓ | mCRPC non-AR driven vs. AR driven ↓ | Biomarker [40,41,42] |
| SORD/Sorbitol dehydrogenase | MDB&PDB vs. LNCaP ↓ | PPARG | AR controlled | PCa vs. NT ↑ | mCRPC vs. PCa ↓ | mCRPC non-AR driven vs. AR driven ↓ | Increased expression in high Gleason PCa; reduced expression after castration [43] | |
| SPON2/Spondin2 | MDB&PDB vs. LNCaP ↓ | HOX13 | AR controlled | mCRPC non-AR driven vs. AR driven ↓ | Biomarker [44,45] | |||
| ATP1B1/Sodium-potassium-transporting ATPase subunit beta1 | PDB vs. LNCaP ↑ ** | PPARG, XBP1, PAX5, HOX13, CMYB | AR controlled | Frequently overexpressed and amplified in CRPC [46] | ||||
| ACTN4/Actinin4 | PDB vs. LNCaP ↓ | PPARG, XBP1, PAX5, HOX13, ZIC2, CMYB | AR coactivator, direct interaction | PCa vs. NT ↓ | Reduced expression in high-grade PCa nuclei [42,47,48] | |||
| HSPB1/HSP27 | PDB vs. LNCaP ↓ | XBP1, PAX5 | AR coactivator, direct interaction | PCa vs. NT & BPH ↑ | PCa vs. NT ↑ | Role in PCa epithelial to mesenchymal transition and resistance [49,50,51] | ||
| KHDRBS1/Sam68 | MDB vs. LNCaP ↓ | PPARG, PAX5, ZIC2, CMYB | AR coactivator/corepressor | Role in regulating AR splice variant, cell proliferation, and survival to chemotherapeutic agents [52,53,54] | ||||
| IMPDH2/Inosine-5-monophosphate dehydrogenase 2 | MDB vs. LNCaP ↓ | PPARG, XBP1, PAX5, HOX13, CMYB | PCa vs. NT ↑ | PCa & mCRPC vs. NT ↑ | PCa vs. mCRPC non-AR driven ↑ | Role in PCa metastasis and progression [55,56] | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capaia, M.; Granata, I.; Guarracino, M.; Petretto, A.; Inglese, E.; Cattrini, C.; Ferrari, N.; Boccardo, F.; Barboro, P. A hnRNP K–AR-Related Signature Reflects Progression toward Castration-Resistant Prostate Cancer. Int. J. Mol. Sci. 2018, 19, 1920. https://doi.org/10.3390/ijms19071920
Capaia M, Granata I, Guarracino M, Petretto A, Inglese E, Cattrini C, Ferrari N, Boccardo F, Barboro P. A hnRNP K–AR-Related Signature Reflects Progression toward Castration-Resistant Prostate Cancer. International Journal of Molecular Sciences. 2018; 19(7):1920. https://doi.org/10.3390/ijms19071920
Chicago/Turabian StyleCapaia, Matteo, Ilaria Granata, Mario Guarracino, Andrea Petretto, Elvira Inglese, Carlo Cattrini, Nicoletta Ferrari, Francesco Boccardo, and Paola Barboro. 2018. "A hnRNP K–AR-Related Signature Reflects Progression toward Castration-Resistant Prostate Cancer" International Journal of Molecular Sciences 19, no. 7: 1920. https://doi.org/10.3390/ijms19071920
APA StyleCapaia, M., Granata, I., Guarracino, M., Petretto, A., Inglese, E., Cattrini, C., Ferrari, N., Boccardo, F., & Barboro, P. (2018). A hnRNP K–AR-Related Signature Reflects Progression toward Castration-Resistant Prostate Cancer. International Journal of Molecular Sciences, 19(7), 1920. https://doi.org/10.3390/ijms19071920