In Vitro Model of Neuroinflammation: Efficacy of Cannabigerol, a Non-Psychoactive Cannabinoid
Abstract
:1. Introduction
2. Results
2.1. CBG Increased Cell Viability in NSC-34 Motor Neurons
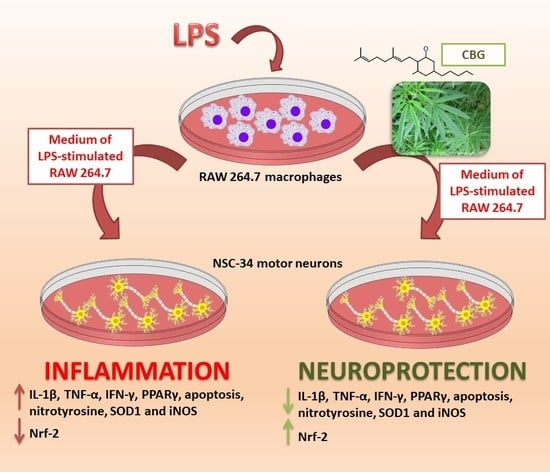
2.2. CBG Counteracted the Loss of Cell Viability and Inhibited Apoptosis in NSC-34 Cells Treated with the Medium of LPS-Stimulated Macrophages
2.3. CBG Reduced the Expression of Pro-Inflammatory Cytokines and Proliferator-Activated Receptor γ (PPARγ)
2.4. CBG Exerted an Antioxidant Action in NSC-34 Cells Treated with Medium of LPS-Stimulated Macrophages
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. General Experimental Procedures
4.3. Extraction and CBG Isolation
4.4. RAW 264.7 Macrophage Cell Culture and LPS Treatment
4.5. NSC-34 Motor Neurons Treatment with the Medium of LPS-Stimulated RAW 264.7
4.6. Thiazolyl Blue Tetrazolium Bromide (MTT) Assay
4.7. Protein Extraction and Western Blot Analysis
4.8. ImmunocytoChemistry
- -
- anti Bax (1:50; Santa Cruz Biotechnology);
- -
- anti Bcl-2 (1:50; Santa Cruz Biotechnology);
- -
- anti IL-1β (1:250; Cell Signaling Technology);
- -
- anti IFN-γ (1:50; Santa Cruz Biotechnology);
- -
- anti TNF-α (1:250; Cell Signaling Technology);
- -
- anti SOD1 (1:100; Abcam, Cambridge, UK);
- -
- anti iNOS (1:50; Santa Cruz Biotechnology);
- -
- anti nitrotyrosine (1:1000; Millipore);
- -
- anti Nrf-2 (1:50; Santa Cruz Biotechnology);
- -
- anti PPARγ (1:50; Santa Cruz Biotechnology).
4.9. Statistical Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Δ9-THC | Δ9-tetrahydrocannabinol |
| CBD | Cannabidiol |
| CBG | Cannabigerol |
| H2O2 | Hydrogen peroxide |
| LPS | Lipopolysaccharide |
| DMSO | Dimethyl sulfoxide |
| PPARγ | Proliferator-activated receptor γ |
| IL-β | Interleukin-1β |
| IFN-γ | Interferon-γ |
| TNF-α | Tumor necrosis factor α |
| iNOS | Inducible nitric oxide synthase |
| SOD1 | Superoxide dismutase1 |
| Nrf-2 | Nuclear factor erythroid 2–related factor 2 |
| PARP-1 | Poly [ADP-ribose] polymerase 1 |
| NF-κB | Nuclear factor-κB |
| MAP | Mitogen-activated protein |
| IL-6 | Interleukin 6 |
| EAE | Experimental Autoimmune Encephalomyelitis |
| NO | Nitric oxide |
| DNBS | Dinitrobenzene sulphonic acid |
| FBS | Fetal bovine serum |
| MTT | Thiazolyl Blue Tetrazolium Bromide |
| HRP | Horse radish peroxidase |
| GAPDH | Glyceraldehyde 3 phosphate dehydrogenase |
| PBS | Phosphate Buffered Saline |
References
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular targets of the phytocannabinoids: A complex picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [PubMed]
- Jackson, S.J.; Diemel, L.T.; Pryce, G.; Baker, D. Cannabinoids and neuroprotection in cns inflammatory disease. J. Neurol. Sci. 2005, 233, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Griffin-Thomas, L. Cannabinoids as therapeutic agents for ablating neuroinflammatory disease. Endocr. Metab. Immune Disord. Drug Targets 2008, 8, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Gowran, A.; Noonan, J.; Campbell, V.A. The multiplicity of action of cannabinoids: Implications for treating neurodegeneration. CNS Neurosci. Ther. 2011, 17, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S. Cannabidiol (cbd) and its analogs: A review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Gugliandolo, A.; Trubiani, O.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Cannabinoid cb2 receptors are involved in the protection of raw264.7 macrophages against the oxidative stress: An in vitro study. Eur. J. Histochem. EJH 2017, 61, 2749. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Di Marzo, V.; et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.G.; Carrillo-Salinas, F.; Pagani, A.; Gomez-Canas, M.; Negri, R.; Navarrete, C.; Mecha, M.; Mestre, L.; Fiebich, B.L.; Cantarero, I.; et al. A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J. Neuroimmune Pharmacol. 2012, 7, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Salinas, F.J.; Navarrete, C.; Mecha, M.; Feliu, A.; Collado, J.A.; Cantarero, I.; Bellido, M.L.; Munoz, E.; Guaza, C. A cannabigerol derivative suppresses immune responses and protects mice from experimental autoimmune encephalomyelitis. PLoS ONE 2014, 9, e94733. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Gomez-Canas, M.; Burgaz, S.; Palomares, B.; Gomez-Galvez, Y.; Palomo-Garo, C.; Campo, S.; Ferrer-Hernandez, J.; Pavicic, C.; Navarrete, C.; et al. Benefits of vce-003.2, a cannabigerol quinone derivative, against inflammation-driven neuronal deterioration in experimental parkinson’s disease: Possible involvement of different binding sites at the ppargamma receptor. J. Neuroinflamm. 2018, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Valdeolivas, S.; Navarrete, C.; Cantarero, I.; Bellido, M.L.; Munoz, E.; Sagredo, O. Neuroprotective properties of cannabigerol in huntington’s disease: Studies in r6/2 mice and 3-nitropropionate-lesioned mice. Neurotherapeutics 2015, 12, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [PubMed]
- Leszek, J.; Barreto, G.E.; Gasiorowski, K.; Koutsouraki, E.; Avila-Rodrigues, M.; Aliev, G. Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: Role of brain innate immune system. CNS Neurol. Disord. Drug Targets 2016, 15, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Weiner, H.L. Cns inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ros in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Mammana, S.; Fagone, P.; Cavalli, E.; Basile, M.S.; Petralia, M.C.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The role of macrophages in neuroinflammatory and neurodegenerative pathways of alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis: Pathogenetic cellular effectors and potential therapeutic targets. Int. J. Mol. Sci. 2018, 19, 831. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Alonso, J.; Paraiso-Luna, J.; Navarrete, C.; Del Rio, C.; Cantarero, I.; Palomares, B.; Aguareles, J.; Fernandez-Ruiz, J.; Bellido, M.L.; Pollastro, F.; et al. Vce-003.2, a novel cannabigerol derivative, enhances neuronal progenitor cell survival and alleviates symptomatology in murine models of huntington’s disease. Sci. Rep. 2016, 6, 29789. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Y.S.; Wang, M.L.; Cheng, C.; Bian, R.; Yuan, H.; Wang, Y.; Guo, T.; Zhu, L.L.; Zhou, H. Protective effect of naringin against the lps-induced apoptosis of pc12 cells: Implications for the treatment of neurodegenerative disorders. Int. J. Mol. Med. 2017, 39, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Giacoppo, S.; Ficicchia, M.; Aliquo, A.; Bramanti, P.; Mazzon, E. Eruca sativa seed extract: A novel natural product able to counteract neuroinflammation. Mol. Med. Rep. 2018, 17, 6235–6244. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; Giacoppo, S.; Trubiani, O.; Diomede, F.; Piattelli, A.; Bramanti, P.; Mazzon, E. Conditioned medium of periodontal ligament mesenchymal stem cells exert anti-inflammatory effects in lipopolysaccharide-activated mouse motoneurons. Exp. Cell Res. 2016, 349, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S. Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cell. Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, J.; Shi, J.; Hou, Y.; Zhu, H.; Zhao, S.; Liu, H.; Ding, B.; Yin, Y.; Yi, G. Increased expression of the peroxisome proliferator-activated receptor gamma in the immune system of weaned pigs after escherichia coli lipopolysaccharide injection. Vet. Immunol. Immunopathol. 2008, 124, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Shi, J.X.; Lu, J.; Che, Z.Q.; Zhu, H.L.; Hou, Y.Q.; Yin, Y.L.; Zhao, S.J.; Ding, B.Y.; Liu, H.M. Up-regulated expression of peroxisome proliferator-activated receptor gamma in the hypothalamic-pituitary-adrenal axis of weaned pigs after escherichia coli lipopolysaccharide challenge. Vet. J. 2010, 184, 230–235. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. An update on ppar activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M.; Yi, H.; Akao, Y.; Tanaka, M. Oxidative stress in mitochondria: Decision to survival and death of neurons in neurodegenerative disorders. Mol. Neurobiol. 2005, 31, 81–93. [Google Scholar] [CrossRef]
- Beal, M.F. Oxidatively modified proteins in aging and disease. Free Radic. Biol. Med. 2002, 32, 797–803. [Google Scholar] [CrossRef]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T. The nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Kazantsev, A.G. The role of nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Johnson, J.A. Nrf2--a therapeutic target for the treatment of neurodegenerative diseases. Free Radic. Biol. Med. 2015, 88, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Tanji, K.; Wakabayashi, K.; Matsuura, S.; Itoh, K. Role of the keap1/nrf2 pathway in neurodegenerative diseases. Pathol. Int. 2015, 65, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinti, L.; Dayalan Naidu, S.; Trager, U.; Chen, X.; Kegel-Gleason, K.; Lleres, D.; Connolly, C.; Chopra, V.; Low, C.; Moniot, S.; et al. Keap1-modifying small molecule reveals muted nrf2 signaling responses in neural stem cells from huntington’s disease patients. Proc. Natl. Acad. Sci. USA 2017, 114, E4676–E4685. [Google Scholar] [CrossRef] [PubMed]
- Xagorari, A.; Roussos, C.; Papapetropoulos, A. Inhibition of lps-stimulated pathways in macrophages by the flavonoid luteolin. Br. J. Pharmacol. 2002, 136, 1058–1064. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugliandolo, A.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. In Vitro Model of Neuroinflammation: Efficacy of Cannabigerol, a Non-Psychoactive Cannabinoid. Int. J. Mol. Sci. 2018, 19, 1992. https://doi.org/10.3390/ijms19071992
Gugliandolo A, Pollastro F, Grassi G, Bramanti P, Mazzon E. In Vitro Model of Neuroinflammation: Efficacy of Cannabigerol, a Non-Psychoactive Cannabinoid. International Journal of Molecular Sciences. 2018; 19(7):1992. https://doi.org/10.3390/ijms19071992
Chicago/Turabian StyleGugliandolo, Agnese, Federica Pollastro, Gianpaolo Grassi, Placido Bramanti, and Emanuela Mazzon. 2018. "In Vitro Model of Neuroinflammation: Efficacy of Cannabigerol, a Non-Psychoactive Cannabinoid" International Journal of Molecular Sciences 19, no. 7: 1992. https://doi.org/10.3390/ijms19071992
APA StyleGugliandolo, A., Pollastro, F., Grassi, G., Bramanti, P., & Mazzon, E. (2018). In Vitro Model of Neuroinflammation: Efficacy of Cannabigerol, a Non-Psychoactive Cannabinoid. International Journal of Molecular Sciences, 19(7), 1992. https://doi.org/10.3390/ijms19071992