Engineering Cell Adhesion and Orientation via Ultrafast Laser Fabricated Microstructured Substrates
Abstract
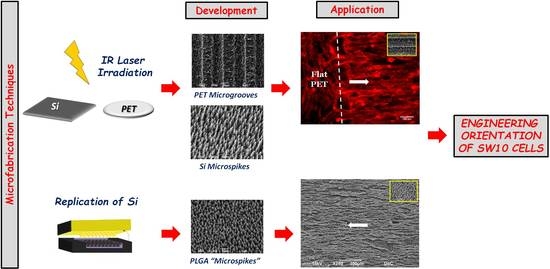
:1. Introduction
2. Results and Discussion
2.1. Scanning Electron Microscopy (SEM) Images of Laser-Microstructured Substrates on PET (PET-MG) and PLGA-MS (1:10) Replicas (from Laser-Microstructured Si Substrates with Three Different Laser Fluences)
2.2. Measurements of Wettability of Irradiated PET (PET-MG), Non-Irradiated PET (PET-Flat), and PLGA-MS (1:10) Replicas (from Irradiated Si Substrates)
2.3. UV–Vis Measurements of Irradiated PET (PET-MG), Non-Irradiated PET (PET-Flat), and PLGA-MS (1:10) Replicas (from Irradiated Si Substrates)
2.4. Cell Seeding of Laser-Microstructured Substrates on PET (PET-MG) and PLGA-MS Replicas with Schwann Cells
2.5. Fluorescent Images of Schwann Cells Seeded on Laser-Microstructured Substrates on PET (PET-MG) (Immunostaining) and PLGA-MS Replicas (Immunostaining)
3. Materials and Methods
3.1. Experimental Setup Used for the Fabrication of Laser-Microstructured Substrates
3.2. Characterization of Laser-Microstructured Substrates
3.2.1. Scanning Electron Microscopy (SEM)
3.2.2. Wettability Measurements of Laser-Microstructured Substrates
3.2.3. UV–Vis Measurements of Laser-Microstructured Substrates
3.3. Cell Culture
3.3.1. Morphology of Schwann (SW10) Cells by Scanning Electron Microscopy (SEM)
3.3.2. Immunocytochemical Assay
3.3.3. Live/Dead Assay
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| A | Absorbance |
| ATR-FTIR | Attenuated Total Reflection-Fourier Transform Infrared (spectroscopy) |
| BSA | Bovine Serum Albumen |
| CO2 | Carbon dioxide |
| CPD | Critical Point Dryer |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| DCM | Dichloromethane |
| DMEM | Dulbecco’s Modified Eagles Medium |
| ECM | Extracellular matrix |
| EthD-1 | Ethidium Homodimer-1 |
| EtOH | Ethanol |
| GDA | Glutaraldehyde |
| HDMS | Hexamethyldisilizane |
| IESL | Institute of Electronic Structure and Laser |
| MG | Microgrooves |
| MS | Microspikes |
| NGF | Nerve Growth Factor |
| PBS | Phosphate-buffered saline |
| PC12 | Pheochromocytoma |
| PDMS | Poly(dimethylsiloxane) |
| PET | Polyethylene terephthalate |
| PFA | Paraformaldehyde |
| PLGA | Poly(lactide-co-glycolide) |
| RT | Room Temperature |
| SCB | Sodium Cacodylate buffer |
| SEM | Scanning Electron Microscopy |
| SF6 | Sulfur hexafluoride |
| Si | Silicon |
| SW10 | Schwann cells |
| UV | Ultraviolet |
| UV-Vis | Ultraviolet-Visible |
| UV/VIS/NIR | Ultraviolet/Visible/Near Infrared |
| Yb:KGW | Ytterbium-doped potassium gadolinium tungstate |
References
- Wu, R.-X.; Yin, Y.; He, X.-T.; Li, X.; Chen, F.-M. Engineering a Cell Home for Stem Cell Homing and Accommodation. Adv. Biosyst. 2017, 1, 1700004. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and Engineering the Cell Surface Interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Pasapera, A.M.; Schneider, I.C.; Rericha, E.; Schlaepfer, D.D.; Waterman, C.M. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol. 2010, 188, 877–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowder, S.W.; Leonardo, V.; Whittaker, T.; Papathanasiou, P.; Stevens, M.M. Material Cues as Potent Regulators of Epigenetics and Stem Cell Function. Cell Stem Cell 2016, 18, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Stukel, J.M.; Willits, R.K. Mechanotransduction of Neural Cells Through Cell–Substrate Interactions. Tissue Eng. Part B Rev. 2016, 22, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yiannakou, C.; Simitzi, C.; Manousaki, A.; Fotakis, C.; Ranella, A.; Stratakis, E. Cell patterning via laser micro/nano structured silicon surfaces. Biofabrication 2017, 9, 25024. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ohlin, C.A.; Lu, Q.; Hu, J. Cell directional migration and oriented division on three-dimensional laser-induced periodic surface structures on polystyrene. Biomaterials 2008, 29, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Rebollar, E.; Frischauf, I.; Olbrich, M.; Peterbauer, T.; Hering, S.; Preiner, J.; Hinterdorfer, P.; Romanin, C.; Heitz, J. Proliferation of aligned mammalian cells on laser-nanostructured polystyrene. Biomaterials 2008, 29, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Ranella, A.; Barberoglou, M.; Bakogianni, S.; Fotakis, C.; Stratakis, E. Tuning cell adhesion by controlling the roughness and wettability of 3D micro/nano silicon structures. Acta Biomater. 2010, 6, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Orgovan, N.; Peter, B.; Bősze, S.; Ramsden, J.J.; Szabó, B.; Horvath, R. Dependence of cancer cell adhesion kinetics on integrin ligand surface density measured by a high-throughput label-free resonant waveguide grating biosensor. Sci. Rep. 2015, 4, 4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarona, E.; Peter, B.; Szekacs, I.; Tsamis, C.; Horvath, R. ZnO Nanostructure Templates as a Cost-Efficient Mass-Producible Route for the Development of Cellular Networks. Materials (Basel) 2016, 9, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simitzi, C.; Karali, K.; Ranella, A.; Stratakis, E. Controlling the Outgrowth and Functions of Neural Stem Cells: The Effect of Surface Topography. ChemPhysChem 2018. [Google Scholar] [CrossRef] [PubMed]
- Simitzi, C.; Ranella, A.; Stratakis, E. Controlling the morphology and outgrowth of nerve and neuroglial cells: The effect of surface topography. Acta Biomater. 2017, 51, 21–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdeen, A.A.; Lee, J.; Kilian, K.A. Capturing extracellular matrix properties in vitro: Microengineering materials to decipher cell and tissue level processes. Exp. Biol. Med. 2016, 241, 930–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Shao, Y.; Li, X.; Zhao, G.; Fu, J. Nanotopographical surfaces for stem cell fate control: Engineering mechanobiology from the bottom. Nano Today 2014, 9, 759–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, H.; Dadsetan, M. Influence of laser surface modifying of polyethylene terephthalate on fibroblast cell adhesion. Radiat. Phys. Chem. 2003, 67, 381–385. [Google Scholar] [CrossRef]
- Dadsetan, M.; Mirzadeh, H.; Sharifi-Sanjani, N.; Salehian, P. IR Laser Surface Modification of Polyethylene Terephthalate as Biomaterial. In Processing and Fabrication of Advanced Materials VIII; World Scientific: Singapore, 2001; pp. 221–229. [Google Scholar]
- Simitzi, C.; Efstathopoulos, P.; Kourgiantaki, A.; Ranella, A.; Charalampopoulos, I.; Fotakis, C.; Athanassakis, I.; Stratakis, E.; Gravanis, A. Laser fabricated discontinuous anisotropic microconical substrates as a new model scaffold to control the directionality of neuronal network outgrowth. Biomaterials 2015, 67, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Simitzi, C.; Stratakis, E.; Fotakis, C.; Athanassakis, I.; Ranella, A. Microconical silicon structures influence NGF-induced PC12 cell morphology. J. Tissue Eng. Regen. Med. 2015, 9, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, E.; Ranella, A.; Fotakis, C. Biomimetic micro/nanostructured functional surfaces for microfluidic and tissue engineering applications. Biomicrofluidics 2011, 5, 13411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, W.N.; Simpson, D.G.; Bigbee, J.W.; Colello, R.J. Evaluating neuronal and glial growth on electrospun polarized matrices: Bridging the gap in percussive spinal cord injuries. Neuron Glia Biol. 2007, 3, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Kim, D.; Mitchel, J.A.; Bellamkonda, R.V. Topography, cell response, and nerve regeneration. Annu. Rev. Biomed. Eng. 2010, 12, 203–231. [Google Scholar] [CrossRef] [PubMed]
- Johansson, F.; Carlberg, P.; Danielsen, N.; Montelius, L.; Kanje, M. Axonal outgrowth on nano-imprinted patterns. Biomaterials 2006, 27, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, S.; Cui, W.; Sherlock, R.; O’connell, C.; Damodaran, G.; Gorman, A.; Windebank, A.; Pandit, A. Effect of functionalized micropatterned PLGA on guided neurite growth. Acta Biomater. 2008, 5, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Rodighiero, S.; Cappelluti, M.A.; Puricelli, L.; Maffioli, E.; Borghi, F.; Negri, A.; Sogne, E.; Galluzzi, M.; Piazzoni, C.; et al. Conversion of nanoscale topographical information of cluster-assembled zirconia surfaces into mechanotransductive events promotes neuronal differentiation. J. Nanobiotechnol. 2016, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Maffioli, E.; Schulte, C.; Nonnis, S.; Grassi Scalvini, F.; Piazzoni, C.; Lenardi, C.; Negri, A.; Milani, P.; Tedeschi, G. Proteomic Dissection of Nanotopography-Sensitive Mechanotransductive Signaling Hubs that Foster Neuronal Differentiation in PC12 Cells. Front. Cell. Neurosci. 2018, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Ripamonti, M.; Maffioli, E.; Cappelluti, M.A.; Nonnis, S.; Puricelli, L.; Lamanna, J.; Piazzoni, C.; Podestà, A.; Lenardi, C.; et al. Scale Invariant Disordered Nanotopography Promotes Hippocampal Neuron Development and Maturation with Involvement of Mechanotransductive Pathways. Front. Cell. Neurosci. 2016, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Zerva, I.; Simitzi, C.; Siakouli-Galanopoulou, A.; Ranella, A.; Stratakis, E.; Fotakis, C.; Athanassakis, I. Implantable vaccine development using in vitro antigen-pulsed macrophages absorbed on laser micro-structured Si scaffolds. Vaccine 2015, 33, 3142–3149. [Google Scholar] [CrossRef] [PubMed]
- Geissler, M.; Xia, Y. Patterning: Principles and Some New Developments. Adv. Mater. 2004, 16, 1249–1269. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, M.; Edalat, F.; Manoucheri, S.; Khademhosseini, A. Engineering microscale topographies to control the cell–substrate interface. Biomaterials 2012, 33, 5230–5246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chollet, C.; Chanseau, C.; Remy, M.; Guignandon, A.; Bareille, R.; Labrugère, C.; Bordenave, L.; Durrieu, M.-C. The effect of RGD density on osteoblast and endothelial cell behavior on RGD-grafted polyethylene terephthalate surfaces. Biomaterials 2009, 30, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, T.; Yang, S.-T.; Kniss, D.A.; Kniss, D.A. Thermal compression and characterization of three-dimensional nonwoven PET matrices as tissue engineering scaffolds. Biomaterials 2001, 22, 609–618. [Google Scholar] [CrossRef]
- Lima, M.J.; Correlo, V.M.; Reis, R.L. Micro/nano replication and 3D assembling techniques for scaffold fabrication. Mater. Sci. Eng. C 2014, 42, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Ahmed, A.; Glennon-Alty, L.; Yang, Y.; Murray, P.; Zhang, H. Patterned substrates fabricated by a controlled freezing approach and biocompatibility evaluation by stem cells. Mater. Sci. Eng. C 2015, 49, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Tay, C.; Pal, M.; Yu, H.; Leong, W.; Tan, N.; Ng, K.W.; Venkatraman, S.; Boey, F.; Leong, D.T.; Tan, L.P. Bio-inspired Micropatterned Platform to Steer Stem Cell Differentiation. Small 2011, 7, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Mandoli, C.; Pagliari, F.; Pagliari, S.; Forte, G.; Di Nardo, P.; Licoccia, S.; Traversa, E. Stem Cell Aligned Growth Induced by CeO2 Nanoparticles in PLGA Scaffolds with Improved Bioactivity for Regenerative Medicine. Adv. Funct. Mater. 2010, 20, 1617–1624. [Google Scholar] [CrossRef]
- Stratakis, E.; Ranella, A.; Fotakis, C. Laser-Based Biomimetic Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2013; pp. 211–236. [Google Scholar]
- Skoulas, E.; Manousaki, A.; Fotakis, C.; Stratakis, E. Biomimetic surface structuring using cylindrical vector femtosecond laser beams. Sci. Rep. 2017, 7, 45114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Donelli, I.; Taddei, P.; Smet, P.F.; Poelman, D.; Nierstrasz, V.A.; Freddi, G. Enzymatic surface modification and functionalization of PET: A water contact angle, FTIR, and fluorescence spectroscopy study. Biotechnol. Bioeng. 2009, 103, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Zorba, V.; Stratakis, E.; Barberoglou, M.; Spanakis, E.; Tzanetakis, P.; Anastasiadis, S.H.; Fotakis, C. Biomimetic Artificial Surfaces Quantitatively Reproduce the Water Repellency of a Lotus Leaf. Adv. Mater. 2008, 20, 4049–4054. [Google Scholar] [CrossRef]
- D’avila, C.; Erbetta, C.; Alves, R.J.; Resende, J.M.; Fernando De Souza Freitas, R.; Geraldo De Sousa, R. Synthesis and Characterization of Poly(D,L-Lactide-co-Glycolide) Copolymer. J. Biomater. Nanobiotechnol. 2012, 3, 208–225. [Google Scholar] [CrossRef]
- Grossetête, T.; Rivaton, A.; Gardette, J.L.; Hoyle, C.E.; Ziemer, M.; Fagerburg, D.R.; Clauberg, H. Photochemical degradation of poly(ethylene terephthalate)-modified copolymer. Polym. (Guildf). 2000, 41, 3541–3554. [Google Scholar] [CrossRef]
- Fechine, G.J.; Rabello, M.S.; Souto Maior, R.M.; Catalani, L.H. Surface characterization of photodegraded poly(ethylene terephthalate). The effect of ultraviolet absorbers. Polym. (Guildf) 2004, 45, 2303–2308. [Google Scholar] [CrossRef]
- Prasad, S.G.; De, A.; De, U. Structural and Optical Investigations of Radiation Damage in Transparent PET Polymer Films. Int. J. Spectrosc. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Upson, S.J.; Partridge, S.W.; Tcacencu, I.; Fulton, D.A.; Corbett, I.; German, M.J.; Dalgarno, K.W. Development of a methacrylate-terminated PLGA copolymer for potential use in craniomaxillofacial fracture plates. Mater. Sci. Eng. C 2016, 69, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Li, N.; Huang, R.; Song, Q.; Wang, L.; Zhang, Q.; Su, R.; Kong, T.; Tang, M.; Cheng, G. The Effects of Topographical Patterns and Sizes on Neural Stem Cell Behavior. PLoS ONE 2013, 8, e59022. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.C.; Chung, E.J.; Ameer, G.A. Advances and Applications of Biodegradable Elastomers in Regenerative Medicine. Adv. Funct. Mater. 2010, 20, 192–208. [Google Scholar] [CrossRef]
- Lietz, M.; Dreesmann, L.; Hoss, M.; Oberhoffner, S.; Schlosshauer, B. Neuro tissue engineering of glial nerve guides and the impact of different cell types. Biomaterials 2006, 27, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Koufaki, N.; Ranella, A.; Aifantis, K.E.; Barberoglou, M.; Psycharakis, S.; Fotakis, C.; Stratakis, E. Controlling cell adhesion via replication of laser micro/nano-textured surfaces on polymers. Biofabrication 2011, 3, 45004. [Google Scholar] [CrossRef] [PubMed]












© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babaliari, E.; Kavatzikidou, P.; Angelaki, D.; Chaniotaki, L.; Manousaki, A.; Siakouli-Galanopoulou, A.; Ranella, A.; Stratakis, E. Engineering Cell Adhesion and Orientation via Ultrafast Laser Fabricated Microstructured Substrates. Int. J. Mol. Sci. 2018, 19, 2053. https://doi.org/10.3390/ijms19072053
Babaliari E, Kavatzikidou P, Angelaki D, Chaniotaki L, Manousaki A, Siakouli-Galanopoulou A, Ranella A, Stratakis E. Engineering Cell Adhesion and Orientation via Ultrafast Laser Fabricated Microstructured Substrates. International Journal of Molecular Sciences. 2018; 19(7):2053. https://doi.org/10.3390/ijms19072053
Chicago/Turabian StyleBabaliari, Eleftheria, Paraskevi Kavatzikidou, Despoina Angelaki, Lefki Chaniotaki, Alexandra Manousaki, Alexandra Siakouli-Galanopoulou, Anthi Ranella, and Emmanuel Stratakis. 2018. "Engineering Cell Adhesion and Orientation via Ultrafast Laser Fabricated Microstructured Substrates" International Journal of Molecular Sciences 19, no. 7: 2053. https://doi.org/10.3390/ijms19072053
APA StyleBabaliari, E., Kavatzikidou, P., Angelaki, D., Chaniotaki, L., Manousaki, A., Siakouli-Galanopoulou, A., Ranella, A., & Stratakis, E. (2018). Engineering Cell Adhesion and Orientation via Ultrafast Laser Fabricated Microstructured Substrates. International Journal of Molecular Sciences, 19(7), 2053. https://doi.org/10.3390/ijms19072053